
Pulmonary Contusion on Admission Chest X-ray is Associated with Coagulopathy and Mortality in Trauma Patients
*Corresponding Author(s):
Charles E WadeDepartment Of Surgery, Center For Translational Injury Research, University Of Texas Health Science Center, Houston, Texas, United States
Tel:+1 7135005391, +1 7135000685
Email:Charles.E.Wade@uth.tmc.edu
Abstract
Introduction: Trauma patients presenting with coagulopathy are four times more likely to die. After observing patients presenting with isolated severe Pulmonary Contusion (PC) on admission Chest X-ray (CXR) who were also coagulopathic, we hypothesized that patients with PC on admission CXR were more likely to be coagulopathic than other trauma patients.
Methods: This was an IRB approved, single-center, retrospective cohort study of patients admitted over an 18 month period. Data were obtained from the trauma registry and medical records including demographics, laboratory values, injury severity scores, coagulation parameters and PC diagnostic modality. Patients on anticoagulants were excluded. The PC group consisted of patients diagnosed with PC by admission CXR. The control group were those patients without a PC or those diagnosed only by CT. Coagulopathy was defined as INR>1.4 or PTT>35 seconds. The PC group was further stratified into mild, moderate or severe PC. Data were analyzed with univariate analyses followed by development of a logistic regression model. Significance was set at p<0.05.
Results: The PC group contained 189 subjects and controls 333. As expected ISS was higher in the PC group (25 vs 21) as was AIS chest (3 vs 1); both p<0.001. The prevalence of coagulopathy was also greater in PC (31% vs. 17%, p<0.001). After adjusting for ISS, PC patients were more likely to be coagulopathic (OR=1.76, 95% CI: 1.12-2.75) and had higher 24 hour (7% vs 3%, p=0.04) and overall mortality rates (15% vs 7%, p=0.006). The prevalence of coagulopathy increased with severity of pulmonary contusion in PC patients (Mild<moderate<severe).
Conclusion: Pulmonary Contusion (PC) present on admission CXR in trauma patients is associated with an increased prevalence of coagulopathy on admission and mortality.
INTRODUCTION
Complications resulting from injury are the leading cause of death and disability worldwide [1,2]. With the exception of central nervous system injury, exsanguination is the most common cause of death following trauma [3]. This fact is particularly important because death resulting from blood loss is potentially preventable. Over the last decade, extensive research efforts and increased funding has been directed at improved understanding of the physiology of hemostasis and coagulopathy in hopes of reducing the overall mortality resulting from hemorrhage. Following severe injury and shock, Trauma Induced Coagulopathy (TIC) has been attributed to multiple mechanisms, including inflammation, shock, tissue trauma, factor dilution, and clotting factor consumption [4]. These mechanisms can interact with one another making coagulopathy a complex and multifactorial complication.
An acute hypocoagulable state has been identified at the time of admission in approximately 25% of severely injured trauma patients [5]. This acute coagulopathy seems to develop before the influence of some traditionally theorized causal mechanisms like dilution, acidosis, or hypothermia, though these mechanisms still represent exacerbating factors [6]. Brohi et al., postulated that acute traumatic coagulopathy is initiated by hypoperfusion and leads to systemic anticoagulation and hyper fibrinolysis via the activated protein C pathway [5,7]. Gando [7] and others [8] have suggested that the pathology develops more from a consumptive coagulopathy consistent with the hemorrhagic DIC phenotype. A recent and possibly unifying theory by Johansson et al., [9,10] proposes that with increasing severity of trauma, there is an increase in sympathoadrenal activation. This neurohumoral response drives coagulopathy by inducing local hemostasis, which could lead to a consumptive coagulopathy, but also activates systemic anticoagulation systems. Whatever the mechanism, these patients presenting with early TIC are up to four times more likely to die [6,11]. As such, early identification of acutely coagulopathic patients or knowledge of which injuries are more likely to lead to coagulopathy is critical to reducing hemorrhage-related mortality. Complicating these efforts to better understand TIC is the possibility that multiple injury mechanisms account for distinct types of coagulopathy and having yet to determine how much contribution varying degrees of shock and tissue injury have on development and severity of TIC.
Pulmonary contusion (PC) is an injury sustained by approximately 17% of patients presenting with multiple injuries [12]. The endothelium is the largest organ in the body (4000 to 7000m2), with the pulmonary endothelium in an adult representing 7 to 9% [1,13]. In the civilian setting, lung contusion most often results from motor vehicle crashes or falls and is reported to be the most common injury resulting from blunt thoracic trauma [14-16]. In the combat setting, PC is likely to result from the passage of projectiles through the parenchyma of the lungs [12,17,18]. The biological plausibility for the present study is that PC results in localized endothelial damage which is a potential source of coagulation disturbances following acute injury.
After several patients presented to our hospital with isolated PC, severe hypoxia, and acute coagulopathy, we decided to investigate the possibility of a relationship between PC and coagulopathy. We hypothesized that patients diagnosed with a PC by their admitting chest X-ray, indicating a more severe injury, are more likely to be coagulopathic in comparison to other trauma patients.
METHODS
This was a single-center study approved by the institutional review board of the University of Texas Health Science Center at Houston and analyzed patients admitted to the Texas Trauma Institute at Memorial Hermann Hospital in Houston, Texas. All patients who were ≥14 years of age, were a highest level of trauma activation and were admitted between August 2009 and February 2011 were included. Patients known to be on anticoagulant therapy by review of electronic medical record were excluded. Data for each patient was obtained from our institution’s Trauma Registry of the American College of Surgeons (TRACS) and from the hospital’s electronic medical record. This information included demographics, initial vital signs, blood gas values, contusion status, severity, and modality of diagnosis (CT or CXR), Injury Severity Score (ISS), Abbreviated Injury Scale (AIS) score by region, mortality, and coagulation parameters as measured by Prothrombin Time (PT), Partial Thromboplastin Time (PTT), and International Normalized Ratio (INR) and rapid thrombelastography. Patients were screened for PC by review of admitting CXR and CT scan reports. Original CXR or CT scan images were viewed and interpreted to score pulmonary contusions based on AIS criteria. Pulmonary contusions are graded according to AIS guidelines. We defined them as mild (AIS=2), moderate (AIS=3), or severe (AIS=4), respectively [19].
Patients were separated into three groups: the study group (PCXR) consisted of those patients with a pulmonary contusion on their initial CXR, those with a contusion identified by CT but not present upon initial CXR and a similar number of patients without a contusion selected randomly from the overall population. Coagulopathy was defined as INR>1.4 or PTT>35 seconds [11]. While they were not used to define the presence of coagulopathy, we also collected rapid Thrombelastography (rTEG) values as an assessment of hemostatic potential [10,20,21]. The r-TEG, similar to standard thrombelastography, generates several values that describe the clotting cascade. The first value generated is the Activated Clotting Time (ACT), which is the time in seconds between initiation of the test and the initial fibrin formation. The k-time is the time needed to reach 20-mm clot strength. The alpha angle is the slope of the tracing that represents the rate of clot formation. The Maximal Amplitude (MA) is the greatest amplitude of the tracing and reflects platelet contribution to clot strength.
Descriptive analyses were performed using ANOVA and Kruskal-Wallis for continuous variables and Pearson chi-square for categorical variables. Binary logistic regression was utilized to create the models with covariates purposefully chosen to adjust for patient injury severity [22]. All analyses were conducted using SPSS statistical software, version 19.0 (SPSS Inc., IL).
RESULTS
Between August 2009 and February 2011, there were 6,920 non-burn adult trauma patients admitted. Of these patients, 1760 patients met inclusion criteria (Figure 1). These patients were screened for pulmonary contusions, resulting in 368 PC patients of whom 5 were excluded due to known use of anticoagulant medication. Of the remaining 363 patients, 189 had a PC on their initial CXR (PC group). One hundred and fifty nine subjects without a contusion were randomly identified as the control subjects. While our original intention was to analyze and compare three groups (patients diagnosed with a PC by CXR, patients diagnosed with PC by CT scan only, and patients without PC), initial analysis of demographic and clinical data showed no difference between those without PC and those with a PC diagnosed only by CT scan. As such, these populations were combined resulting in the control population (NCXR; n=333).
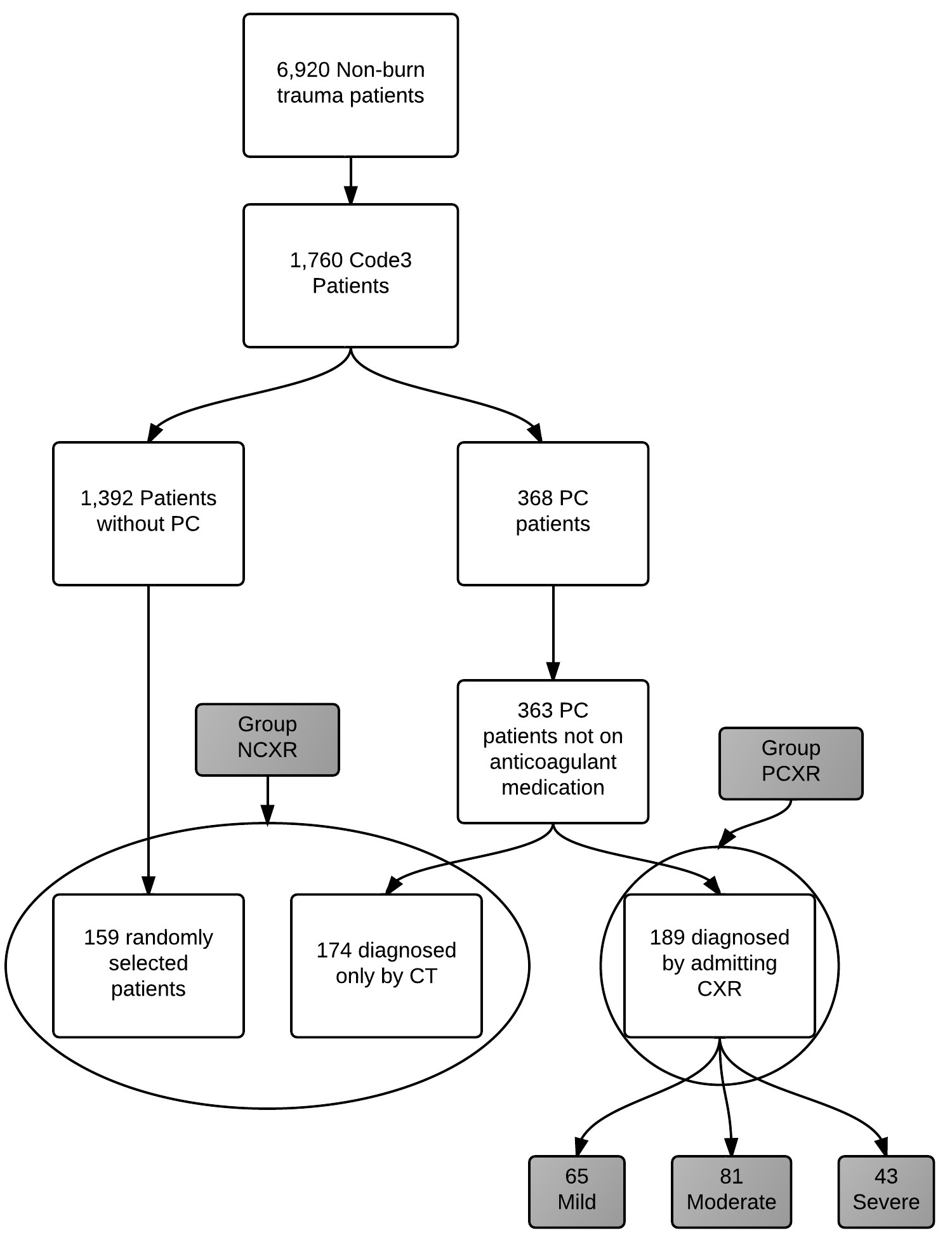 Figure 1: Representation of study design and group assignment.
Figure 1: Representation of study design and group assignment.
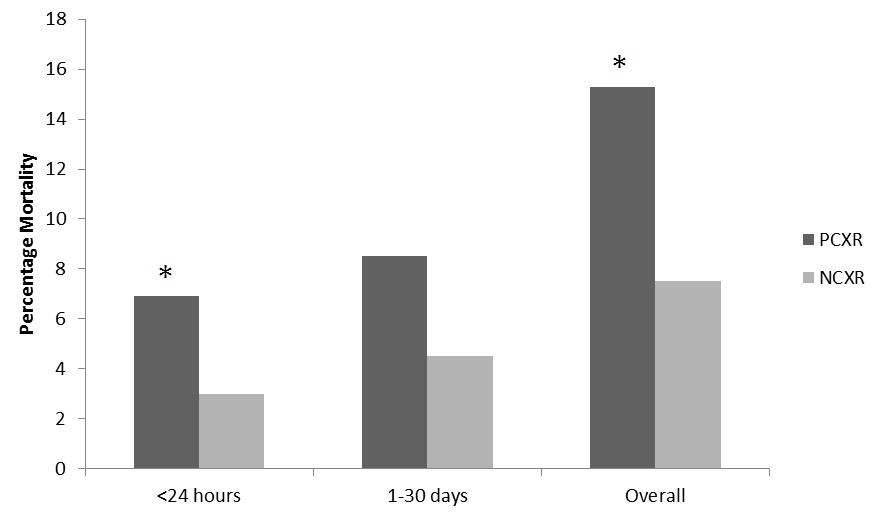
Admission vital signs, laboratory values, and ISS are presented in table 1. PC patients presented more tachycardic and tachypneic. In addition, AIS chest and overall ISS were higher in the PC group compared to controls. Coagulation and laboratory values for both groups are displayed in table 2. The PC group had a worse base deficit, more prolonged PT and PTT and increased INR on arrival. Differences in TEG parameters are indicative a delay in the onset of clotting (ACT value) and a reduction in clot strength (alpha angle and MA) in the PCXR group. In the PCXR group there was a higher rate of mortality in the first 24 hours and overall (Figure 2).
| PCXR | NCXR | |
| N | 189 | 333 |
| Age (yr) | 33 (16.3) | 36 (16.8) |
| % Blunt | 79 | 75 |
| HR (beat/min) | 107 (26.8) | 101 (26.0)* |
| RR (beat/min) | 24 (9.0) | 21 (6.3)* |
| SBP (mmHg) | 119 (31.1) | 123 (32.8) |
| GCS | 8 (5.46) | 9.3 (5.6)* |
| ISS | 25 (14.34) | 21 (14.29)* |
| AIS Head | 0 (0.4) | 2 (0.4) |
| AIS Chest | 3 (3.4) | 0 (0.2)* |
| AIS Abdomen | 0 (0.3) | 0 (0.2) |
| AIS Extremity | 2 (0.3) | 2 (0.3) |
Table 1: Descriptive comparisons between patients with a pulmonary contusion on their initial CXR and those without a pulmonary contusion on CXR (n=522). Values are mean ± SD or median (IQR) where appropriate.
* p≤0.01.
| PCXR | NCXR | |
| N | 189 | 333 |
| PLT count (103/ml) | 240 (73.5) | 250 (70.4) |
| HGB (g/dl) | 13.0 (2.0) | 13.2 (2.2) |
| pH | 7.2 (0.1) | 7.3 (0.1)** |
| BE | -5 (5.0) | -3 (5.1)* |
| PT (sec) | 16 (3.3) | 15 (2.6)** |
| INR | 1.4 (0.7) | 1.2 (0.4)** |
| PTT (sec) | 32 (11.2) | 28 (7.3)** |
| TEG Values | ||
| ACT (sec) | 124 (34.6) | 119 (17.2)* |
| K (min) | 1.9 (1.8) | 2.0 (5.83) |
| Alpha (degrees) | 69 (8.9) | 72 (5.9)** |
| MA (mm) | 61 (8.3) | 63 (6.5)** |
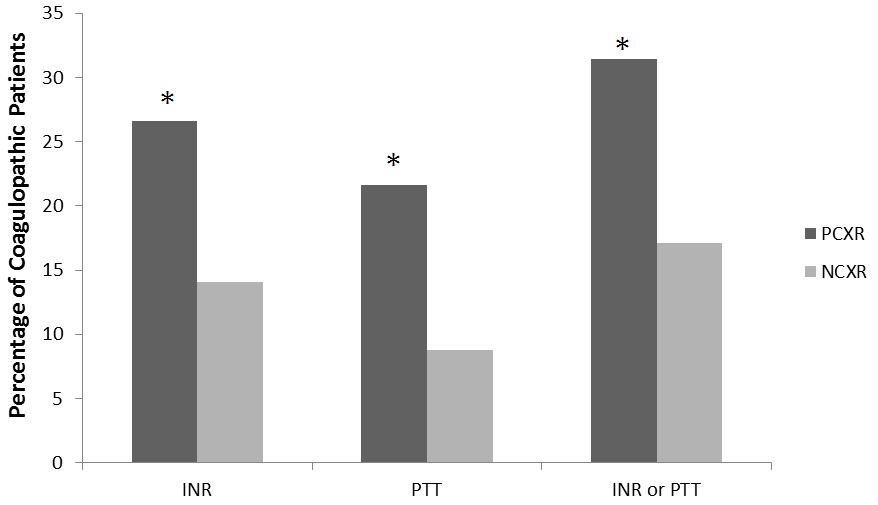
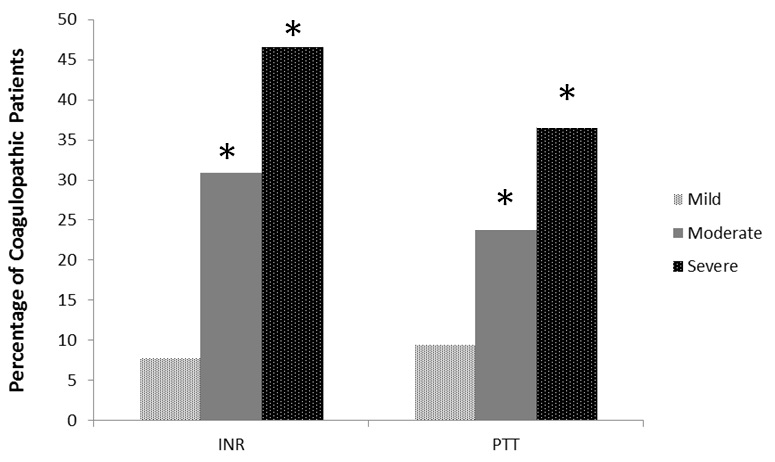
After determining that PCXR group patients were more likely to experience coagulopathy and transfusion requirements, additional analysis was performed on these patients. Of patients in PCXR group the severity of the contusions were, 34% mild, 43% moderate, and 23% severe. The more severe the contusion in PC group patients, the longer the mean coagulation times and the higher percentage of patients experiencing coagulopathy (Table 3 and figure 4).The difference between the percentages of patients that were coagulopathic by INR or PTT between PCXR and NCXR were significant (Figure 3). The unadjusted odds ratios for coagulopathy upon hospital admission in the PC group was two to three fold higher compared to trauma patients with no contusions (INR>1.4, OR=2.21, 95% CI 1.41-3.48 and PTT>35, OR 2.86, 95% CI 1.69-4.82). After adjusting for ISS, patients diagnosed with a contusion by CXR (PCXR group) were more likely to be coagulopathic by either INR or PTT (OR=1.76, 95% CI 1.12-2.75, p=0.01). Furthermore, patients with PC were more likely to receive Red Blood Cell (RBC) transfusions than those with no contusions (53% vs. 42%, p=0.02). Also if they received a transfusion they received more RBC units over the first 24 hours than the NCRX group (4 units vs. 2 units, on average, p=0.01).
|
Mild (n = 65) |
Moderate (n = 81) |
Severe (n = 43) |
P - value | |
| INR | 1.21±0.25 | 1.4±0.43 | 1.73±1.29 | <0.001 |
| PTT (sec) | 29.2±0.25 | 30.9±9.45 | 37.9±15.1 | <0.001 |
DISCUSSION
The major impetus for conducting this study was the observation of several patients presenting with an isolated pulmonary contusion, profound hypoxia and significant coagulopathy. While the literature is extensive on both pathologies, there is little regarding a relationship between them. A literature review for PC and coagulopathy revealed no directed studies in humans, though previous studies identified weak associations [15,18]. There was a relevant experiment conducted by Batchinsky et al., [2] that induced PC in swine to elucidate the mechanism by which it led to hypoxemia. This study also looked for any changes in coagulation parameters as measured by PT and a PTT. Experimental contusion in swine caused mildly decreased clot initiation and formation rate, but these changes were not accompanied by an increase in PT or activated PTT. With limited human or animal data available, the process of developing the current study was initiated.
One of the initial challenges faced in designing this study was how to measure the severity and significance of a pulmonary contusion in a way that is relevant to and quickly reproducible in clinical practice. A reliable and automated method of quantifying pulmonary pathology using CT scans has been developed by Daly et al., [23]. However the software and time investment that would be required to analyze each CT scan, even in a semi-automated fashion, would reduce the applicability of this research in the case of severely injured patients where time is critical. Although CT scans are both more sensitive and specific for detecting PC [24] they are not always immediately available, take longer to obtain than plain films, and can be limited by poor respiratory effort in injured patients. Additionally, due to the superior sensitivity of CT as opposed to CXR, some patients will be correctly diagnosed with a PC that might be clinically insignificant. AIS scoring guidelines for assessing and comparing PC severity, while useful in retrospective review, are frequently calculated too late to be useful in the acute presentation and also may require a CT. A CXR will miss smaller contusions and will not give as much detail as a CT but, broadly speaking, a CXR will detect the more clinically important injuries. CXR also has the advantage of being quick and readily available in most situations. For these reasons we chose to separate the groups by presence or absence of a pulmonary contusion on admitting CXR.
Since there is a well-known association between increasing injury severity and coagulopathy, and there was a difference between the ISS scores of the groups, an adjustment was made for ISS in our regression model [25]. With respect to anatomic regions, the only AIS region where a difference existed between the PC and control groups was AIS Chest (3.2 for PC and 1.5 for controls. Importantly, there was no difference in the AIS Head scores which could have been a confounding factor since head injury as it has also been associated with coagulopathy [26]. In light of these findings we feel the ISS difference between the groups was the result of the more severe chest injury in the PC patients. As described above, having a PC diagnosed on initial CXR increased the likelihood of coagulopathy on admission. The current study we noted that the degree of coagulopathy followed pulmonary contusion grade in a dose-dependent fashion, with more serious contusions leading to both a higher frequency of and more severe coagulopathies. Johansson and co-workers [10,21] have put forth the hypothesis that endothelial damage is a function of the severity of injury initially promoting systemic coagulation activation, and that the hypocoagulability seen subsequently an evolutionary response to counteract the initial response to injury. Thus, we proposed major damage to the lungs, an organ with an extensive endothelium, would lead to a greater incidence of hypocoagulation. Our findings may serve as biological plausibility for localized pulmonary endothelial damage as a potential source of coagulation disturbances following acute injury. This may be related to the volume of injured pulmonary endothelium and the known interaction between the endothelium and coagulopathy. Of note while there were significant differences in BE between subjects with PC and controls based on blood pressure and the subsequent low amount of RBCs administered it is assumed that the coagulopathy was from pulmonary injury rather than the systemic insult associated with hypo-perfusion.
Due to the design of the study, we believe our results are broadly and easily applicable to the practicing clinician. Coagulopathy is a life threatening entity, especially when it manifests early in the treatment of traumatically injured patients. One of the first tests performed on many trauma patients is a CXR and the results of such films are usually quickly available. If a pulmonary contusion is detected on the admitting CXR of a trauma patient, particularly a severe contusion, this finding should be a clue to the physician to be wary of the patient’s coagulation status and increased mortality.
As a retrospective investigation, this study has several limitations. This is a single center study evaluating the most severely injured patients at a tertiary referral center. Though resuscitation fluids and products have been shown to effect coagulation status, we did not collect data on prehospital interventions nor did we exclude transfers. In addition, our definition of coagulopathy by INR or PTT may not be standard and these tests suffer from their ability to detect coagulopathy in non-anticoagulated patients. A randomized controlled trial would offer the best evidence, but this is not feasible. However, a prospective observational design, assessing more data points and following patients in a serial fashion, would strengthen evidence for a relationship and could also be helpful in identification of a mechanism. More research is needed to fully understand how pulmonary contusion, hypoxia and coagulopathy are related.
CONCLUSION
Severe pulmonary contusions are more likely to be diagnosed by an admitting CXR. Patients diagnosed with a contusion on admitting CXR are more likely to experience coagulopathy, an increased need for RBC transfusions, and mortality when compared to other trauma patients even after adjusting for ISS differences. The prevalence and severity of coagulopathy increases with severity of pulmonary contusion. Physicians detecting pulmonary contusion on initial CXR should be concerned about the risk of coagulopathy and mortality for their patients.
REFERENCES
- Bender DA, Bender AE (1999) Bender’s Dictionary of Nutrition and Food Technology, (7thedn). Taylor & Francis, UK.
- Batchinsky AI, Jordan BS, Necsoiu C, Dubick MA, Cancio LC (2010) Dynamic changes in shunt and ventilation-perfusion mismatch following experimental pulmonary contusion. Shock 33: 419-425.
- Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, et al. (1995) Epidemiology of trauma deaths: a reassessment. J Trauma 38: 185-193.
- Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, et al. (2008) The coagulopathy of trauma: a review of mechanisms. J Trauma 65: 748-754.
- Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, et al. (2008) Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma 64: 1211-1217.
- Brohi K, Cohen MJ, Davenport RA (2007) Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care 13: 680-685.
- Gando S, Sawamura A, Hayakawa M (2011) Trauma, shock, and disseminated intravascular coagulation: lessons from the classical literature. Ann Surg 254: 10-19.
- Hayakawa M, Sawamura A, Gando S, Kubota N, Uegaki S, et al. (2011) Disseminated intravascular coagulation at an early phase of trauma is associated with consumption coagulopathy and excessive fibrinolysis both by plasmin and neutrophil elastase. Surgery 149: 221-230.
- Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR (2011) A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg 254: 194-200.
- Johansson PI, Ostrowski SR (2010) Acute coagulopathy of trauma: balancing progressive catecholamine induced endothelial activation and damage by fluid phase anticoagulation. Med Hypotheses 75: 564-567.
- MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M (2003) Early coagulopathy predicts mortality in trauma. J Trauma 55: 39-44.
- Cohn SM (1997) Pulmonary contusion: review of the clinical entity. J Trauma 42: 973-979.
- Aird WC (2007) Endothelial Biomedicine. Cambidge University Press, UK.
- Crankson SJ, Fischer JD, Al-Rabeeah AA, Al-Jaddan SA (2001) Pediatric thoracic trauma. Saudi Med J 22: 117-120.
- Jin H, Li-Qun Tang, Zhi--Guo Pan, Na Peng, Qiang Wen, et al. (2014) The-Year induced endothelial activation and damage by fluid phase anticoagulation. Medical hypothesis.
- Klein Y, Cohn SM, Proctor KG (2002) Lung contusion: pathophysiology and management. Curr Opin Anaesthesiol 15: 65-68.
- Edens JW, Chung KK, Pamplin JC, Allan PF, Jones JA, et al. (2010) Predictors of early acute lung injury at a combat support hospital: a prospective observational study. J Trauma 69: 81-86.
- Keneally R, Szpisjak D (2013) Thoracic trauma in Iraq and Afghanistan. J Trauma Acute Care Surg 74: 1292-1297.
- Gennarelli TA, Wodzin E (2006) AIS 2005: a contemporary injury scale. Injury 37: 1083-1091.
- Cotton BA, Faz G, Hatch QM, Radwan ZA, Podbielski J, et al. (2011) Rapid thrombelastography delivers real-time results that predict transfusion within 1 hour of admission. J Trauma 71: 407-414.
- Holcomb JB, Minei KM, Scerbo ML, Radwan ZA, Wade CE, et al. (2012) Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Ann Surg 256: 476-486.
- Bursac Z, Gauss CH, Williams DK, Hosmer DW (2008) Purposeful selection of variables in logistic regression. Source Code Biol Med 3: 17.
- Daly M, Miller PR, Carr JJ, Gayzik FS, Hoth JJ, et al. (2008) Traumatic pulmonary pathology measured with computed tomography and a semiautomated analytic method. Clin Imaging 32: 346-354.
- De Wever W, Bogaert J, Verschakelen J (2000) Radiology of lung trauma. JBR-BTR 83: 167-173.
- Frith D, Davenport R, Brohi K (2012) Acute traumatic coagulopathy. Curr Opin Anaesthesiol 25: 229-234.
- Laroche M, Kutcher ME, Huang MC, Cohen MJ, Manley GT (2012) Coagulopathy after traumatic brain injury. Neurosurgery 70: 1334-1345.
Citation: Wright AP, Wade CE, Camp E, Caga-Anan Z, Radwan ZA, et al. (2015) Pulmonary Contusion on Admission Chest X-ray is Associated with Coagulopathy and Mortality in Trauma Patients. J Emerg Med Trauma Surg Care 2: 011.
Copyright: © 2015 Adam P Wright, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

