
An Evaluation of the Preventive Role of Ficus religiosa on High Fat Diet and Low Dose Streptozotocin Induced Diabetes Mellitus in Rats
*Corresponding Author(s):
Rajendra NathDepartment Of Pharmacology, King George’s Medical University, Lucknow, Uttar Pradesh, India
Tel:+91 9454615783,
Fax:+91 5222257448
Email:rajendra.nath79@gmail.com
Abstract
Objective
To evaluate the protective role of Ficus religiosa in the development of diabetes and inflammation in high fat diet and low dose Streptozotocin (STZ) induced diabetic rat models.
Materials and methods
30 male adult Wistar rats were divided into 5 groups (n = 6). Rats in group I were fed normal pellet diet for 4 weeks and were taken as normal control. Rats in groups II-V were first fed with high fat diet for 4 weeks and were then given an intraperitoneal injection of low dose of Streptozotocin (30 mg/kg body weight). Group II was taken as diabetic control while rats in groups III-V were treated with Ficus religiosain a dose of 100, 200, and 400 mg/kg body weight respectively. Body weight, fasting plasma glucose, and plasma Tumor Necrosis Factor (TNF)-α levels were measured for evaluation.
Results
Ficus religiosa in all the three doses was found to be effective in preventing a significant increase in fasting plasma glucose while it was effective in preventing a significant increase in body weight and plasma tumor necrosis factor-α level in a dose of 400 mg/kg body weight.
Conclusion
Treatment with Ficus religiosa shows protection against elevation of fasting plasma glucose and plasma Tumor necrosis factor -α level in rats exposed to risk factors like high fat diet and streptozotocin and suggest a protective role in the development of diabetes mellitus.
Keywords
INTRODUCTION
Diabetes mellitus is an ‘iceberg’ disease, as a large number of individuals who meet the current criteria for diabetes mellitus remain asymptomatic and unaware of having this disorder. Once regarded as a single disease entity, diabetes is now seen as a heterogenous group of diseases, characterized by a state of chronic hyperglycemia resulting from a diversity of etiologies, both environmental and genetic, acting jointly. Depending on the etiology, factors contributing to this state of chronic hyperglycemia include reduced insulin secretion, decreased glucose utilization, and increased glucose production. These metabolic dysregulations further cause secondary pathophysiologic changes in multiple organ systems which impose a tremendous burden on the individual with diabetes and also on the national health care system [1]. Studies in recent years have shown that chronic low-grade inflammation, more specifically inflammatory cytokines (mainly IL 1, TNF-α, and IL 6) [2] also contribute to the development of type 2 diabetes mellitus and its microvascular complications [3-5].
Diabetes mellitus has recently been identified by Indian Council of Medical Research (ICMR) as one of the refractory diseases for which satisfactory treatment is not available in modern allopathic system of medicine. Furthermore, allopathic system do not have any safer and effective drug which could reduce the risk or delay the development of diabetes mellitus in high risk individuals. WHO (1980) has also recommended the evaluation of the effectiveness of plants in conditions where there are no safe modern drugs [6]. Moreover the diabetes mellitus type-2 is increasing as an epidemic in developed and developing countries across the world and there is no satisfactory preventive treatment except the life style changes.
It is therefore evident to harness and harvest medicinal plants having multiple beneficial effects which can not only take care of most of the metabolic abnormalities associated with diabetes [7,8] but can also reduce the risk of developing or delay the onset of diabetes mellitus amongst susceptible persons. Therefore in search of effective alternative therapy we have taken the plant herb Ficus religiosa having multiple beneficial medicinal effects [9-11] but the work for its role in diabetes mellitus type-2 is in infancy.
The present study was therefore envisaged to discern the protective role of Ficus religiosa in the development of diabetes and inflammation in high fat diet and low dose streptozotocin induced diabetic rat models, which has not been evaluated so far.
MATERIALS AND METHODS
Experimental animals
Diet
Drugs and chemicals
Route of drug administration
Experimental protocol
Group I/ Ficus religiosa Control (FRC, n = 6): Ficus religiosa (200 mg/kg) + normal pellet diet
Group II/ Diabetic Control (DC, n = 6): Distilled water + High Fat Diet (HFD)
Group III (n = 6): Ficus religiosa (100 mg/kg) + HFD
Group IV (n = 6): Ficus religiosa (200 mg/kg) + HFD
Group V (n = 6): Ficus religiosa (400 mg/kg) + HFD
A baseline body weight of all rats was recorded on day 0 of the study and blood sample was drawn for baseline biochemical evaluation of fasting plasma glucose and plasma TNF-α levels.
After 4 weeks of treatment, the body weight of rats in group I was recorded and blood sample was withdrawn for fasting plasma glucose levels while rats in groups II- V were given an intraperitoneal injection of a low dose (30 mg/ kg body weight) of Streptozotocin (STZ) for induction of type 2 diabetes mellitus [14-16] which is as follows:
Group II/ Diabetic Control (Distilled water and HFD treated group) + STZ (30 mg/kg )
Group III, IV & V (Ficus religiosa and HFD treated group) + STZ (30 mg/kg)
One week after STZ injection i.e., at the end of the 5th week of the study, body weight of rats of groups II- V was recorded and blood sample was drawn for biochemical evaluation of fasting plasma glucose and plasma TNF-α levels.
BIOCHEMICAL ANALYSIS
Fasting plasma glucose level
Estimation of plasma TNF-α
STATISTICAL ANALYSIS
All values were expressed as means ± SD. The data was analyzed by one way Analysis of Variance (ANOVA) and one-sample Kolmogorov-Smirnov test. Independent t test/ Tuke’s pairwise comparison was used for comparison between the treatment groups. Differences in treatment effects within groups and between the treatment and control groups were tested by a multivariate analysis of variance repeated-measures design with treatment type as a between-subject factor (2 groups) and treatment effect (baseline compared with follow-ups) as a within-subject factor. Statistical significance was based on a two-tailed p value < 0.05.
OBSERVATIONS AND RESULTS
Baseline study
| Variables | Controls | Treatment Groups | |||
|
Group I (FRC) |
Group II (DC) |
III (FR 100 mg/kg) | IV (FR 200 mg/kg) | V (FR 400 mg/kg) | |
| BW | 227.3 ± 26.1 | 227.3 ± 25.9 | 203.5 ± 10.1 | 212.3 ± 29.9 | 201.5 ± 32.7 |
| FPG | 126.8 ± 13.0 | 90.7 ± 17.2 | 84.8 ± 14.3 | 102.7 ± 15.5 | 108.7 ± 15.6 |
| TNF-α | 4.68 ±1.04 | 5.5 ± 1.8 | 6.3 ± 0.5 | 4.7 ± 2.1 | 7.2 ± 1.8 |
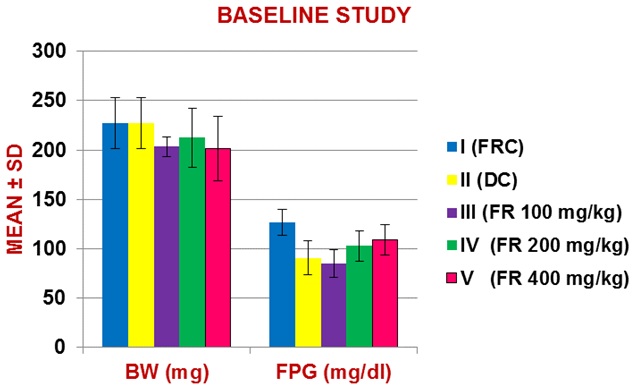
Figure 1: Baseline (day 0) Mean (± SD) body weight and Fasting Plasma Glucose (FPG, mg/dl) levels in 5 groups of rats (n=6).
Effect of Ficus religiosa on normal rats
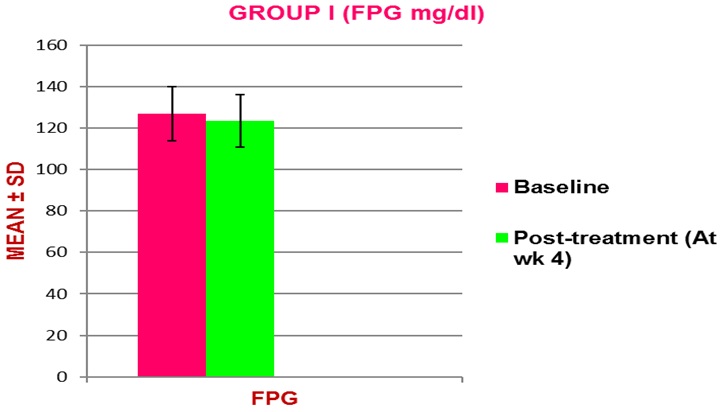
Figure 2: Baseline and post-treatment mean (± SD) Fasting Plasma Glucose (FPG) in normal rats (group I).
Effect of Ficus religiosa on biochemical markers
| Variables | Groups | Periods | Baseline to Week 5 | ||
| Baseline (day 0) | Post-treatment (week 5) | % mean change | p-value1 | ||
| Body Weight | II | 227.3 ± 25.9 | 257.8 ± 1.9 | 6.11 | 0.03* |
| III | 203.5 ± 10.1 | 243.3 ± 7.1 | 19.87 | 0.001* | |
| IV | 212.3 ± 29.9 | 247.3 ± 26.1 | 17.98 | 0.04* | |
| V | 201.5 ± 32.7 | 213.8 ± 17.0 | 11.32 | 0.46 | |
| FPG | II | 90.7 ± 17.1 | 184.7 ± 17.4 | 107.03 | 0.001* |
| III | 84.8 ± 14.3 | 101.2 ± 15.5 | 22.63 | 0.15 | |
| IV | 102.7 ± 15.5 | 111.8 ± 30.7 | 13.06 | 0.58 | |
| V | 108.7 ± 15.6 | 105.5 ± 4.4 | -2.83 | 0.73 | |
| TNF-α | II | 5.5 ± 1.8 | 7.6 ± 1.3 | 47.49 | 0.002* |
| III | 6.3 ± 0.5 | 7.4 ± 0.7 | 35.96 | 0.03* | |
| IV | 4.7 ± 2.1 | 5.0 ± 2.1 | 28.93 | 0.04* | |
| V | 7.2 ± 1.8 | 6.0 ± 0.9 | 26.46 | 0.83 | |
1Paired t-test, *Significant
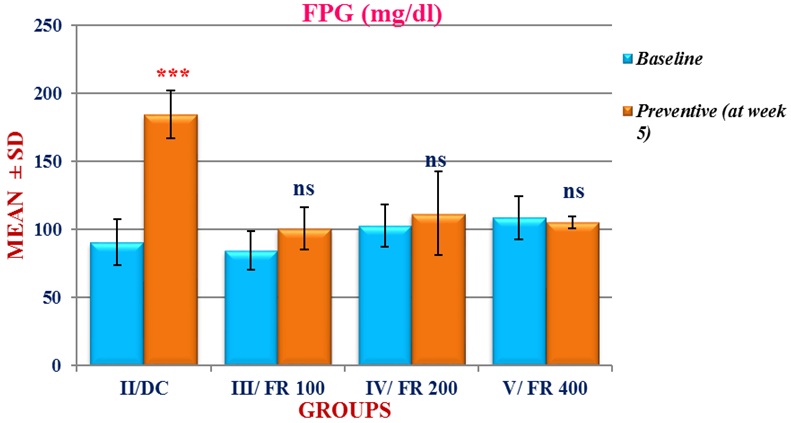
Figure 3: Baseline and Post-treatment mean (± SD) Fasting Plasma Glucose (FPG) in four groups of rats (groups II-V).
***p< 0.001 (highly significant); ns: p > 0.05 (not significant)
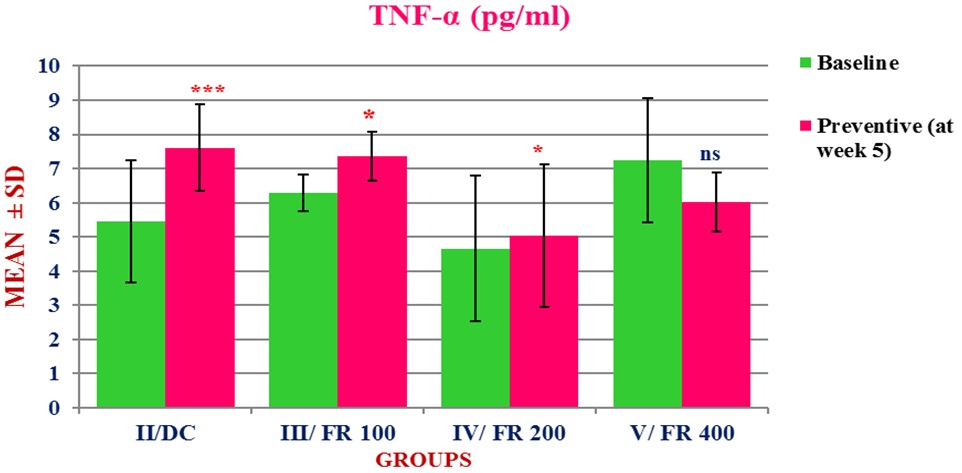 Figure 4: Baseline and Post-treatment mean (± SD) plasma TNF-α in four groups of rats (groups II-V).
Figure 4: Baseline and Post-treatment mean (± SD) plasma TNF-α in four groups of rats (groups II-V).***p< 0.001 (highly significant); *p< 0.05 (significant); ns: p> 0.05 (not significant)
TNF-α level was significantly increased from baseline to week 5 (5.46 ± 1.79 to 7.61 ± 1.27, p = 0.002) in group II. Table 2, depicts the average percent change in the level of TNF-α from baseline to week 5 in four groups, II-V.
Body weight was significantly increased from baseline (day 0) to week 5 in Groups II (p = 0.03), III (p = 0.001), and IV (p = 0.04).
There was an increase in the level of FPG from baseline to week 5 in groups II-IV. Fasting Plasma Glucose (FPG) level was significantly increased from baseline to week 5 (90.67 ± 17.15 to 184.67 ± 17.41, p = 0.001) in Group II. Table 2, depicts the average percent change in the level of FPG from baseline to week 5 in groups II-V.
DISCUSSION AND CONCLUSION
| Variables | Groups | Baseline | At week 5 | |||
| Mean difference | p-value | Mean difference | p-value | |||
| FPG | Gr II vs | III | 5.83 | 0.99 | 83.5 | <0.001* |
| IV | 12 | 0.91 | 72.83 | <0.001* | ||
| V | 18 | 0.53 | 79.16 | <0.001* | ||
| TNF- α | Gr II vs | III | 0.83 | 0.99 | 0.24 | 1 |
| IV | 0.8 | 0.99 | 2.57 | 2.57 | ||
| V | 1.78 | 0.61 | 1.59 | 0.64 |
Insulin resistance is one of the most important risk factors associated with type 2 diabetes and is polygenic and multifactorial in origin. Chronic low grade inflammation and activation of innate immunity as underlying factors in the pathogenesis of insulin resistance and type 2 diabetes mellitus is also now well established. Various studies have suggested that elevated levels of inflammatory markers (mainly IL 1, TNF-α, and IL 6) are independent predictors of type 2 diabetes mellitus [17,18]. TNF-α may cause insulin resistance through the following possible mechanisms: a) Direct inhibitory effect on glucose transporter protein GLUT4, insulin receptor substrates, or glucose-stimulated insulin release by pancreatic β-cells, or b) Indirect effect by increasing free fatty acid oxidation, stimulation of insulin counter-regulatory hormones or cytokines [e.g., IL-6, TNF-α and C-Reactive Protein (CRP), or impairment of endothelial function.
Modern allopathic drugs till date have not been a concrete solution in reducing the risk of developing diabetes mellitus or delaying the onset of diabetes mellitus in susceptible individuals. It is therefore evident to look for options that belong to safer indigenous system of medicines. Ficus religiosa is known to have multiple beneficial effects and has been extensively used in traditional medicine for a wide range of ailments of the central nervous system, endocrine system, gastrointestinal tract, reproductive system, respiratory system, and infectious disorders. However, this plant has not been studied for its preventive effect in diabetes mellitus. With the ayurvedic concept of management in mind we have chosen to evaluate the effect of Ficus religiosa in the prevention of development of diabetes mellitus in the present study.
Effect of Ficus religiosa on normal rats (Group I, Ficus religiosa 200 mg/kg body weight)
PREVENTIVE STUDY
Groups III, IV, and V were evaluated for the preventive effects of Ficus religiosa in the development of diabetes mellitus in susceptible subjects exposed to risk factors like high fat diet and low dose STZ. They were given Ficus religiosa in a dose of 100, 200, and 400 mg/kg body weight respectively along with High Fat Diet (HFD) for 4 weeks. We used the purified dried powder form in our study as powder form usually contains most of the active compounds. Moreover it is a safer form to consume as a drug as compared to other forms like extract.
Toxicity study to determine the safe oral dose of Ficus religiosa was not done in our study because acute toxicity tests, as per Organisation for Economic Co-operation and Development (OECD) 423 guidelines (2010) have already been conducted in many previous studies. Khan et al., [19] (2010) reported that methanolic extract of Ficus religiosa did not produce any acute toxicity, or significant behavioral change, or mortality up to an oral dose of 2000 mg/ kg in male Wistar rats.
As compared to baseline, the body weight in diabetic control group i.e. group II (p=0.03) and in groups III (p = 0.001) and IV (p = 0.04) showed a significant increase while the increase in body weight of group V was not found to be statistically significant (Table 2).
On comparing the results between the three treatment groups, the percent increase in mean body weight was found to be least in group V (+ 11.32%) followed by group IV (+ 17.98%), with a maximum increase in group III (+ 19.87%) (Table 2). These results suggest that Ficus religiosa at a higher dose (400 mg/kg) may have a protective effect in preventing high fat diet induced weight gain (Table 2).
As compared to baseline, although the FPG at week 5 showed an increase in groups II, III, and IV, but the increase was found to be statistically significant only in group II (Table 2). In group V, a decrease in FPG was observed which was statistically not significant. When compared with group II, the FPG values of groups III, IV, and V were found to be significantly (p<0.001) less (Table 2 and Figure 3). Thus, as hypothesized in our study, Ficus religiosa in all the three doses was found to be effective in preventing a significant increase in FPG after 4 weeks of HFD f/b STZ injection.
If we see the comparison between the three treatment groups (i.e., III-V) then it was observed that though both groups III and IV did not show a significant increase in FPG from baseline, but the percent mean increase from baseline in group III (+ 22.63 %) was higher than group IV (+ 13.06 %) (Table 2). The best response was however seen in group V, which did not cause any increase in FPG levels from baseline.
We may thus infer from these findings that although all the three doses of Ficus religiosa were effective in preventing the development of a state of hyperglycemia, but Ficus at a dose of 400 mg/kg was found to be more effective than 200 mg/kg which in turn had a better effect than at a dose of 100 mg/kg (Table 2).
Plasma TNF-α level at week 5 increased significantly in group II (p = 0.002), III (p =0.03), and IV (p = 0.04) as compared to baseline (Table 2 and Figure 4). However, an insignificant increase in TNF-α levels was observed in group V at week 5 as compared to the baseline (Table 2). This suggests that Ficus in a dose of 400 mg/kg was effective in preventing a significant increase in TNF-α levels.
As compared to the control group, which showed a + 47.49% mean increase from baseline, the percent mean increase in group III, IV, and V was found to be + 35.96%, + 28.93%, and + 26.46% respectively (Table 2). We may therefore conclude from these results that although only group V treatment could succeed in preventing a significant increase in TNF-α levels, but the remaining two preventive groups (III and IV) also managed to cause a less increase as compared to the control (group II). Thus, as hypothesized in our study, Ficus religiosa was found to be effective in preventing a significant increase in TNF-α level.
Since no comprehensive planned study has been done so far to see the preventive effect of Ficus religiosain the development of diabetes in rats exposed to risk factors like HFD and STZ, our findings could find no resemblance to the recent literature.
Keeping in view the results obtained in the present study, the following conclusions may be drawn regarding the potential protective role of Ficus religiosa against development of diabetes mellitus and pro-inflammatory markers:
• Ficus religiosa did not produce any significant hypoglycemic effect in normal rats, therefore it may be prescribed to normal individuals prophylactically.
• Ficus religiosa in all the three doses i.e., 100, 200, and 400 mg/kg b.w., is effective in preventing the onset of hyperglycemia when exposed to risk factors like high fat diet. A dose dependent response was observed with different doses used. The dose of 400 mg/kg lead to the most pronounced effect.
• Ficus religiosa in a dose of 400 mg/kg had a significant preventive effect against the elevation of inflammatory marker TNF-α as well.
In light of the above evidences, Ficus religiosa may be recommended prophylactically along with proper diet and exercise for preventing the onset of diabetes mellitus. The results of the present study are encouraging and may reveal the importance of Ficus religiosa as an economical prophylactic agent but details of the complete mechanism have yet not been explored. Therefore, further experiments are required to elucidate the exact mechanism of action. Also, more specific and longer duration animal and human studies are required to further substantiate the findings of the present study.
ETHICAL CLEARANCE
This work has been approved by Institutional Animal Ethical Committee (IAEC) and registered under Registration No. 646/02/a//CPCSEA for conducting animal tissue experiment.
It was funded by a university research grant.
REFERENCES
- Powers AC (2008) Diabetes Mellitus. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, et al. (eds.). Harrison’s Principles of Internal Medicine. 17th edn, McGraw Hill, New York, USA. Pg no: 2275-2304.
- Alexandraki K, Piperi C, Kalofoutis C, Singh J, Alaveras A, et al. (2006) Inflammatory process in type 2 diabetes: The role of cytokines. Ann N Y Acad Sci 1084: 89-117.
- Jeffcoate WJ, Game F, Cavanagh PR (2005) The role of proinflammatory cytokines in the cause of neuropathic osteoarthropathy (acute Charcot foot) in diabetes. Lancet 366: 2058-2061.
- Mocan MC, Kadayifcilar S, Eldem B (2006) Elevated intravitreal interleukin-6 levels in patients with proliferative diabetic retinopathy. Can J Ophthalmol 41: 747-752.
- Mora C, Navarro JF (2006) Inflammation and diabetic nephropathy. Curr Diab Rep 6: 463-468.
- Upadhayay UP, Pandey K (1984) Ayurvedic approach to Diabetes mellitus in developing countries. In: Bajaj JS (ed.). New Delhi, India. Pg no: 375-377.
- Kameswararao B, Giri R, Kesavulu MM, Apparao C (1997) Herbal medicines: In the treatment of diabetes mellitus. Manphar Vaidya Patrika, India 1: 33-35.
- Rizvi SI, Mishra N (2013) Traditional Indian medicines used for the management of diabetes mellitus. J Diabetes Res 2013: 712092.
- Verma N, Kumar D, Chaudhary AK, Singh R, Tyagi S (2012) Antidiabetic potential of methanolic bark extract of Ficus religiosa L. in Streptozotocin induced diabetic rats. Int Jour of Biopharmaceutical & Toxicological Research 2: 261-265.
- Chaturvedi N, Shukla K, Singh A (2014) Post-prandial glucose response to Ficus religiosa based products in normal subjects and their outcome on glycemic index. Int Journal of Advanced Research 2: 219-226.
- Gautam S, Meshram A, Bhagyawant SS, Srivastava N (2014) Ficus religiosa-potential role in pharmaceuticals. IJPSR 5: 1616-1623.
- Pandit R, Phadke A, Jagtap A (2010) Antidiabetic effect of Ficus religiosa extract in streptozotocin-induced diabetic rats. J Ethnopharmacol 128: 462-466.
- Kirana H, Jali MV, Srinivasan BP (2011) The study of aqueous extract of Ficus religiosa Linn. on cytokine TNF-α in type 2 diabetic rats. Pharmacognosy Res 3: 30-34.
- Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett JG, et al. (2000) A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism 49: 1390-1394.
- Wang HJ, Jin YX, Shen W, Neng J, Wu T, et al. (2007) Low dose Streptozotocin (STZ) combined with high energy intake can effectively induce type 2 diabetes through altering the related gene expression. Asia Pac J Clin Nutr 16: 412-417.
- Wang B, Li Y, Liu X, Liu S, Sun C (2011) [Effect of the duration of high-fat diet and the dosage of streptozotocin on establishing experimental animal model of type 2 diabetes mellitus]. Wei Sheng Yan Jiu 40: 99-102,106.
- Miles PD, Romeo OM, Higo K, Cohen A, Rafaat K, et al. (1997) TNF-alpha-induced insulin resistance in vivo and its prevention by troglitazone. Diabetes 46: 1678-1683.
- Hu FB, Meigs JB, Li TY, Rifai N, Manson JE (2004) Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes 53: 693-700.
- Khan MSA, Hussain SA, Jais AMM, Zakaria ZA, Khan M (2011) Anti-ulcer activity of Ficus religiosastem bark ethanolic extract in rats. Journal of Medicinal Plants Research 5: 354-359.
Citation: Rathi P, Pant KK, Dixit RK, Kumar A, Pal R, et al. (2016) An Evaluation of the Preventive Role of Ficus religiosa on High Fat Diet and Low Dose Streptozotocin Induced Diabetes Mellitus in Rats. J Diabetes Metab Disord 3: 012.
Copyright: © 2016 Priyanka Rathi, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

