
Aortoesophageal Fistula: Rare Cause of Gastrointestinal Bleeding
*Corresponding Author(s):
Ludovit DanihelIiird Surgical Clinic, Faculty Of Medicine, Comenius University In Bratislava, Slovakia
Tel:00421 904 331 928,
Email:l.danihel@gmail.com
Abstract
Upper gastrointestinal hemorrhage is a common clinical symptom of various diseases of esophagus, stomach and duodenum. Aortoesophageal fistula is considered a rare but particularly serious condition, which can lead to a series of life-threatening complications. The causes of this lesion are markedly different, ranging from atherosclerotic and inflammatory changes of the aorta to tumorous lesions of esophagus or primary tumors of mediastinum. Chiari (1914) defined triad of classical clinical signs characteristic for this critical condition which include mid-thoracic pain, dysphagia followed by a sentinel hemorrhage and later fatal hematemesis. In this case report, we review a case of an 85-year-old male patient admitted with symptoms of gastrointestinal hemorrhage, who passed with the signs of massive upper gastrointestinal bleeding 2 hours after admission, in spite of intensive therapeutic care. This case highlights the importance of regarding aortoesophageal fistula in the differential diagnosis of upper gastrointestinal bleeding together with the diagnostic pitfalls and therapeutic challenges of this entity.
Keywords
Aortic Atherosclerosis; Aortoesophageal Fistula; Gastrointestinal Hemorrhage
INTRODUCTION
Massive upper gastrointestinal (GI) bleeding is a serious and often fast paced pathological condition, which may in a short time lead to life threatening complications. The causes of upper GI bleeding are diverse. The most common causes of massive hemorrhage include esophageal varices in the terrain of advanced liver cirrhosis, acute and chronic gastroduodenal ulcers, aggressive malignant neoplasms of esophagus and stomach and arteriovenous malformations of the large vessels of aortic arch or vessels of upper GI tract and respiratory tract.
Aortoesophageal fistula (AEF) represents a rare cause of upper GI bleeding. The first mention of AEF in literature dates to 1818 when Dubrueil published a case of AEF caused by ingestion of foreign body [1]. Almost hundred years later, in 1914, Chiari defined triad of classical clinical signs accompanying this dangerous condition [2]. In 1918 Funk described an autopsy case of ruptured AEF in male patient suffering from syphilis [3]. It was not until 1969 when Yonago et al reported a first successful surgical correction of AEF [4], followed by numerous published successful treatments of AEF in the coming years [5-8].
CASE REPORT
85-year-old Caucasian male with a history of stage III hypertension (after ESH/ESC classification), ischemic heart disease, chronic atrial fibrillation and universal atherosclerosis was admitted to our hospital with symptoms of massive hemoptysis and suspicion of pulmonary neoplasm. Previously patient suffered by myocardial infarction as well as ischemic brain stroke. Upon the admission patient presented with dyspnea, anemia and complained about painful deglutition and retrosternal chest pain. Emergency flexible esophagogastroscopy did not uncover the source of bleeding. In spite of intensive hemostyptic therapy, there was recurrence of massive hemoptysis, and 2 hours after admission patient died with signs of hemorrhagic shock.
The autopsy uncovered marked aortic atherosclerosis with numerous fibrous and calcified plaques affecting up to 70 % of the surface of aorta. The posterior wall of left ventricle revealed scar after myocardial infarction reaching the size of 3×2 cm and 5 mm thickness. The brain showed signs of microscopic lacunar infarctions in white matter and basal ganglia. Distal to the main branches of aortic arch, in the aortic convexity, saccular aneurysm was present. The aneurysm reached 6 cm in diameter with evident intimal defect extending through the aortic wall reaching the length of 25 mm (Figure 1).
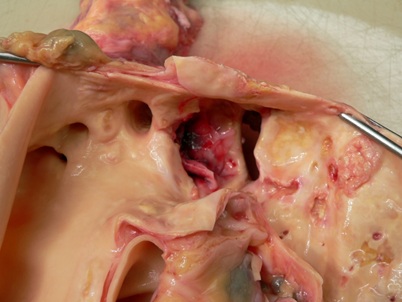 Figure 1: Aneurysm of the aorta with rupture.
Figure 1: Aneurysm of the aorta with rupture.
The outer surface of aneurysm was adhering to the parietal pleura in the upper left pleural cavity. The aneurysmal sack was filled with multiple thrombi in different stages of organization (Figure 2).
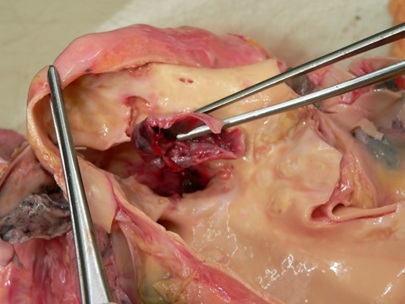 Figure 2: The cavity of aneurysm filled with thrombotic material.
Figure 2: The cavity of aneurysm filled with thrombotic material.
The adhesions between pleura and aneurysm together with the thrombi filling the aneurysmal sack formed covered perforation of the aneurysm. The presence of different stages of thrombotic organization visible during the histological examination indicated a process that was lasting several weeks to months. From the aneurysm lead irregular fistula, 4 mm in diameter, with multiple branches heading ventromedial and caudal direction through the soft tissues of mediastinum (Figure 3).
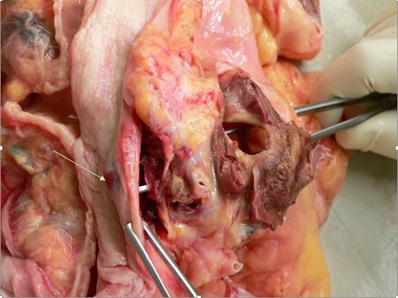 Figure 3: Aortoesophageal fistula (arrow–esophagus with fistula).
Figure 3: Aortoesophageal fistula (arrow–esophagus with fistula).
The fistula was filled with liquid blood and opened into the middle portion of esophagus on the left side, forming a red pouch 8 mm in diameter with 1.5 mm mucosal tear visible on the esophageal surface (Figure 4).
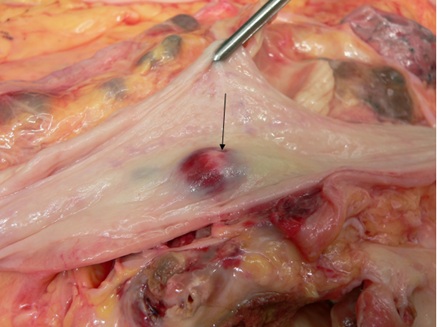 Figure 4: Lumen of esophagus with fistula (arrow).
Figure 4: Lumen of esophagus with fistula (arrow).
The stomach was filled with 550 g of coagulated blood, with additional 350 ml of liquid blood present in small intestines. Mucosa of stomach and duodenum was without any defects. Histologic examination of aorta proved extensive atherosclerotic changes in the form of atheromatous plaques as well as exulcerated plaques. Microscopic examination of esophagus at the site of tear showed necrosis of the wall and mucosa of esophagus with visible perforation.
DISCUSSION
Aortoesophageal fistula is an uncommon, but extremely serious pathological condition with often fatal outcome [8]. The problematic diagnosis of this lesion arises from often asymptomatic development of aortic aneurysm that leads in the case of rupture to sudden severe bleeding to the pleural or pericardial cavity but in some instances can also burst into bronchial or esophageal lumen as well as into large vessels of mediastinum [7,9]. The first sign of rupture might be sentinel bleeding from the upper GI tract. According to Carter, who published in 1978 results from 24 autopsies of patients with aortic aneurysm, the formation of AEF with subsequent rupture is a rare complication of aortic aneurysm appearing in 6.2-22.1% of cases. This study also determined that the most common area of AEF formation (in 75 %) is rupture of aorta in the thoracic region [10].
AEF typically presents with classical clinical symptoms known as Chiari triad that include middle chest pain, dysphagia and sentinel hemorrhage followed by exsanguinating hematemesis [2]. Apart from typical clinical development the diagnosis can be supported by endoscopical examination of upper part of GI tract, which should confirm the source of bleeding. If AEF is present, there should be submucosal hematoma evident at the site of fistula opening to the esophagus. In the case of uncontrolled bleeding it is crucial, apart from intensive care and administration of transfusion therapy, to apply Sengstaken-Blakemore tube. In the stable condition of patient it is advisable to perform computed tomography scan which yields great results in patients with acute bleeding. However, during bleeding-free interval the routing of the fistula might not be always evident. Patients in unstable condition should immediately undergo emergency surgery.
In the case of accidental or timely diagnosis of AEF the emergency surgical intervention might be possible with application of thoracic endovascular aortic repair (TEVAR) by a stent graft [6] or by replacing defective aorta with aortic prosthetic graft [5,7]. For endovascular procedure to take place, it is better that the patient is hemodynamically stable with possible access site from both inguinal areas through retrograde femoral artery catheterization. This technique has an application also in a setting of hemodynamically unstable patients, however, the implementation in this case depends to a great extent on the organization within the workplace as well as on the expertise of endovascular surgeon or interventional radiologist and anatomical relations of each patient. The literature recommends minimum 1.5 cm distance from the lesion for safe fixation of the prothesis. Considering the seriousness of the diagnosis, it is necessary to evaluate the overall health status of the patient, bearing in mind risks and benefits of curative intervention or palliative procedure with possibility of long-lasting antibiotic therapy. The possible complications resulting from the intervention include spinal ischemia and prosthesis endoleaks. Other possible important complications include stroke, especially in patients with proximally located lesion or in patients who have already suffered a stroke before or have a thrombus in the aortic arch [11]. Potential complications of endovascular procedure include among others also paraplegy, visceral ischemia and post-implantation syndrome with febrilities and leukocytosis.
In complicated cases the esophageal wall is repaired in second sitting. If the remaining fistula has a large diameter, it is advisable to endoscopically insert vacuum sponge system to the cavity (Endo-sponge), which is changed every 3 days until the granulation of the remaining fistula is completed. The intervention, surgical or endoscopic, carries a lot of possible complications, however, it is important to bear in mind that it is the only option improving the possible survival of patients.
The hemorrhage resulting from rupture of AEF is a very dangerous and often missed condition with fast and dramatic development which in spite of timely intensive care often ends with fatal outcome, however, in the case of early detection the survival of patients is possible through rapid surgical intervention.
REFERENCES
- Dubrueil (1818) Observations súr la perforation of l´oesophage et de l´aortae thoracique par une potion d´os avale: avec der reflexions. J Univ Sci Med 9: 357-363.
- Chiari H (1914) Ueber Fremdkörperverletzung des Öesophagus mit Aortenperforation. Berl Klin Wschr 51: 7-9.
- Funk EH (1918) Aortic aneurysm with eosophageal rupture. Med Clin North Am 2: 795-802.
- Yonago RH, Iben AB, Mark JBD (1969) Aortic bypass in the management of aortoesophageal fistula. Ann Thorac Surg 7: 235-237.
- Da Silva ES, Tozzi FL, Otochi JP, de Tolosa EMC, Neves CRB, et al. (1999) Aortoesophageal fistula caused by aneurysm of the thoracic aorta: successful treatment, case repost, and literature review. J Vasc Surg 30: 1150-1157.
- Xu R, Li D, Zhu Z, Xuan Ch, Zan W, et al. (2013) Surgical approach for the treatment of aortoesophageal fistula combined with dual aortic aneurysms: a case report. J Cardiothorac Surg 8: 206.
- Kieffer E, Choche L, Gomes D (2003) Aortoesophageal fistula: value of in situ aortic allograft replacement. Ann Surg 238: 283-290.
- Amin S, Luketich J, Wald A (1998) Aortoesophageal fistula: case report and review of the literature. Dig Dis Sci 43: 1665-1671.
- Biskerstaff LK, Pairolero PC, Hollier LH, Melton LJ, Van Peenen HJ, et al. (1982) Thoracic aortic aneurysms: a population based study. Surgery 92: 1103-1108.
- Carter R, Mulder GA, Snyder EN, Brewer LA III (1978) Aortoesophageal fistula. Am J Surg 136: 26-28.
- Gutsche JT, Cheung AT, McGarvey ML, Moser WG, Szeto W, et al. (2007) Risk factors for perioperative stroke after thoracic endovascular aortic repair. Ann Thorac Surg 84: 1195-1200.
Citation: Danihel L, Sisovsky V, Mosna K, Schnorrer M (2019) Aortoesophageal Fistula: Rare Cause of Gastrointestinal Bleeding. J Emerg Med Trauma Surg Care 6: 038.
Copyright: © 2019 Ludovit Danihel, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

