
Celecoxib Decreases Gcf Prostaglandin E2 Levels in Chronic Periodontitis Patients
*Corresponding Author(s):
Athena S PapasTufts University School Of Dental Medicine, Boston, MA, United States
Tel:+1 6176363932,
Email:athena.papas@tufts.edu
Abstract
Background: Prostaglandin E2 (PGE2) is a key mediator associated with periodontal bone loss. We recently demonstrated (Yen, et al, J Periodontology 79:104, 2008) that adjunctive use of Celecoxib (a COX-2 selective inhibitor) in conjunction with Scaling and Root Planning (SRP) resulted in significant reduction in Pocket Depth (PD) and gain in Clinical Attachment (CAL) in Chronic Periodontitis (CP) patients. The purpose of this study was to explore any association between PGE2 levels in the Gingival Crevicular Fluid (GCF) and clinical periodontal signs in a subset of subjects who took part in a double-blind, placebo-controlled, randomized clinical trial investigating the effects of Celecoxib (COX-2) on CP.
Material and methods: Sixty-four subjects were randomly selected from the total subject pool of 131. After a comprehensive periodontal examination, subjects were randomized to receive either 200mg Celecoxib (COX-2, n = 35) or placebo (PLA, n = 29) once/day for 6 months in conjunction with SRP. Probing Depth (PD) and Clinical Attachment Level (CAL) were recorded and GCF samples were collected from 6 different teeth (one site per tooth) at three time points (baseline, 3 months and 6 months). Concentrations of PGE2 in GCF samples were measured using enzyme-linked immunosorbent assay.
Results: PD and CAL improved significantly over the 6 month period. There was a modest positive relationship between PD and PGE2 (Spearman rank correlation: r = 0.12, p = 0.037). Probing depth was analyzed using one way analysis of variance. Any decrease in PD was compared to no decrease. In the Celebrex group, those who had a decrease in PD from baseline to 6 months showed a statistically significant drop in PGE2 levels, when compared to those who did not decrease their PD (172.8 pg/ml vs. 97.7 pg/ml, p = 0.01). The COX-2 group demonstrated a statistically significant decrease in PGE2 levels from baseline to 3 months compared to the placebo group (65.5 pg/ml vs. 13.5 pg/ml, p = 0.013), but not at 6 months (109.8 pg/ml vs. 71.2 pg/ml, p = 0.12).
Conclusion: These results suggest that Celecoxib (COX-2) in conjunction to SRP significantly reduces PGE2 levels in the GCF in the first 3 months post treatment in CP patients. This decrease is associated with improved clinical outcomes (investigator-initiated study funded by Pfizer).
Keywords
Anti-inflammatory agents; Cyclooxygenase inhibitors; Cyclooxygenase 2; Non-steroidal; Periodontitis
INTRODUCTION
Periodontal disease is associated with variable microbial patterns, systemic diseases, local and environmental predisposing factors. Development of periodontal disease was believed to depend on the interaction between the resident oral microbiota, found in the dento-gingival plaque, and the host response. The bacteria host interaction activates a sequence of host immune mechanisms in an effort to achieve homeostasis even at the expense of damaging periodontal tissues. However, emphasis on the role that specific microbes play in chronic inflammatory periodontitis has changed. No longer all periodontal infections are the same; thus, they should not be treated the same. Today, from a pathologic perspective, understanding of periodontitis is shifting toward a more individualized chronic inflammatory response dependent on different biologic factors. Offenbacher et al., proposed a biologic system to define periodontal disease based on the clinical phenotype, biological phenotype which includes molecular, cellular and microbial differences, genetic and epigenetic composition, and personal level exposures such as smoking, diabetes, obesity, and biofilm. Greater understanding of these complex pathways involved in inflammation may be the future therapeutic strategy to combat inflammation and chronic diseases potentially arising from it [1,2]. Even though multiple components contribute to periodontal disease, most of the tissue damage is caused by the host response to infection via the numerous tissue mediators released in response to noxious stimuli [3]. The progression and magnitude of the periodontal disease is mediated by major mediators such as Prostaglandin (PG), histamine, leukotrienes, lysosomal products, lymphokines, 5-hydroxytryptamine, macrophage products, kinins, complements, and clotting factors [4,5]. PG’s are found in almost all nucleated cells and most tissues and organs. Phospholipase degrades the phospholipids in the plasma membrane of cells, releasing free Arachidonic Acid (AA). The intermediate AA is created from Diacylglycerol (DGLA) Via Phospholipase-A2, then brought to either the Cyclooxygenase (COX) or lipoxygenase pathway to form prostaglandin and thromboxane or leukotriene respectively. The cyclooxygenase pathway produces thromboxane, prostacyclin and prostaglandin D, E and F. COX-1 is responsible for the baseline levels of prostaglandins as shown in Figure 1.
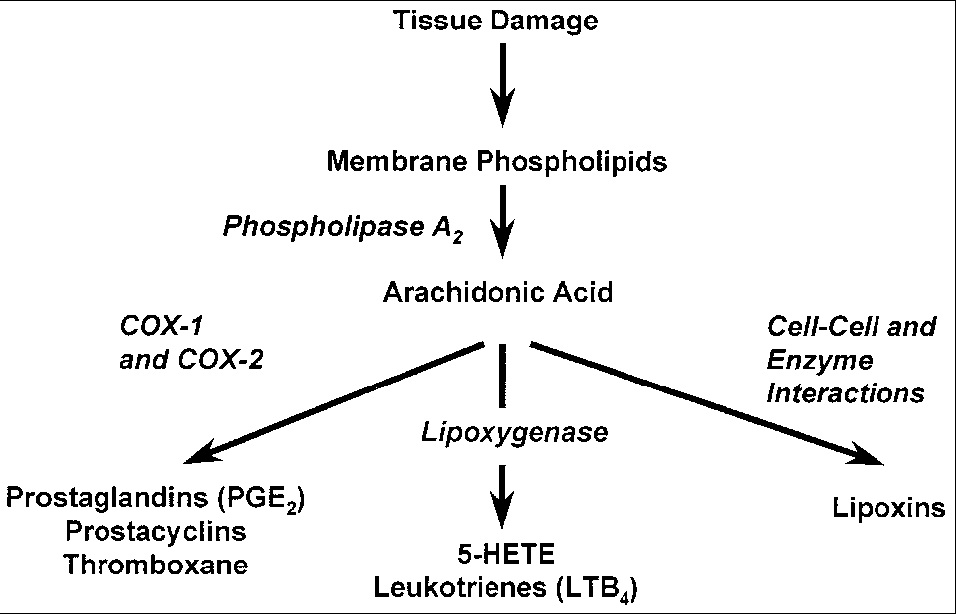 Figure 1: The cyclooxygenase pathway.
Figure 1: The cyclooxygenase pathway.
The COX enzyme is formed by two isoforms (COX-1 and COX-2). The pattern of tissue expression and regulation is the major difference between the two COX isoforms. COX-1 is expressed constantly in almost all tissue and its expression increases 2-4 folds under stimulatory conditions. However, COX-2 is undetectable in normal conditions but its expression increases 10-80 fold locally due to stimulus from inflammatory cytokines, growth factors, or endotoxins. COX-2 produces prostaglandin in scenarios of inflammation [6]. Various periodontal studies in the 70’s and 80’s by Goodson, El Attar et al., and Offenbacher determined that levels of PGE2 in inflamed gingiva were 10-fold higher than that in healthy gingiva or were 20 times elevated in diseased adult human gingival tissues [7,8]. These metabolites of arachidonic acid pathway have been implicated in a wide range of events that are associated with disease such as platelet aggregation, vasodilatation, vasoconstriction, chemotaxis of neutrophils, increased vascular permeability, and bone resorption and closely associated with gingivitis and alveolar bone resorption. PGE2 is of particular interest because 1) Prostaglandins have been demonstrated to be potent stimulators of bone résorption in tissue culture [9]; 2) PGE2 levels within the periodontal tissues and gingival crevicular fluid (GCF) are elevated in periodontal disease and correlate with clinical expression of the disease [7,8,10,11]; and 3) NSAIDs reduce bone loss in animals and humans with periodontal disease [12,13].
According to Therman O’Brien et al., studies with Immunoreactive Prostaglandin E2 (iPGE2) and Immunoreactive Leukotriene B4 (iLTB4) in patients post periodontal surgery treated with NSAIDs or not, PGE2 is an important mediator/predictor of pain and inflammation in periodontal tissues post surgery and that the action of prostaglandins can be inhibited through the use of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) [14-16]. Therefore, Gingival Crevicular Fluid (GCF) PGE2 levels can be used as a reliable biochemical predictor of inflammation and positive predictor of periodontal disease activity in chronic periodontitis patients [1,3]. However, sole use of PGE2 GCF values for disease monitoring makes it difficult due to extensive variability in concentrations, host-to-host variation, and complexity associated with inflammation, and technique sensitivity. Identifications and development of test to better diagnose and monitor disease pathways and periodontitis is an area of continuous research and expands in the efforts of identifying a set of markers or patterns that increase our predictability and development of new novel more targeted therapies with minimal side effects.
Periodontal treatment historically has involved mechanical disruption of plaque and calculus associated with the tooth and the periodontal pocket [15,16]. In this study we considered the inhibitory effects of adjunctive pharmacological therapy with Non-Steroidal Anti-Inflammatory Drugs (NSAID’s) COX-2 inhibitor/ Celecoxib on GCF levels of PGE2 in patients with chronic periodontitis. In addition, a small pool of samples were analyzed using LINCOplex™ human cytokine kit to simultaneously screen samples simultaneous multi analyte detection and measurement.
MATERIALS AND METHODS
Study design
This was a double blind, prospective, randomized, placebo controlled human clinical study of 12-month duration. The protocol was approved by the Tufts New England Medical Center Institutional Review Board. FDA approval was obtained for this investigator instigated study. After being assessed for eligibility and periodontal status at screening and baseline visits, study subjects were randomly assigned to receive either Celecoxib 200mg or placebo once a day for 6 months. The study subjects received SRP in all four quadrants in two visits and were assessed clinically during periodontal maintenance every three months for a period of one year.
Study population
Recruited 131 adult (age¨18-75) patients with evidence of CP over 1 year period. Sixty-four subjects were randomly selected from the total subject pool of 131 for the PGE2 GCF analysis. Although our power analysis is based on a sample size of 120 patients, it is anticipated that up to 150 patients will enter the study during the initial 6 months, in order to take into account possible attrition and non-compliance. After the 6 month mark, no further subject recruitment is planned.
Inclusion criteria
Each patient had a minimum of 16 teeth, at least two of which were molars. At least four teeth with probing depths of > 4mm and loss of attachment > 2mm were present, with a minimum of two interproximal areas with radiographic evidence of bone loss.
Exclusion criteria
History of periodontal therapy (other than prophylaxis) in the past 12 months; long-term use of aspirin or NSAIDs within the previous 12 months; history of antibiotic therapy in the past 6 months; any condition that would require antibiotic pre-medication for prevention of subacute infective endocarditis; hypersensitivity to Celecoxib or other COX-2 inhibitors, aspirin or NSAIDs; history of radiation therapy in the head and neck area; history of Sjogren’s syndrome; history of peptic ulcers or any other condition that would be a contraindication for the use of NSAIDs or COX-2 inhibitors (aspirin/NSAID-induced asthma or urticaria; aspirin triad, hepatic failure, sulfa drug allergy, pregnancy, labor and delivery, nursing, men fathering children).
Conditions that are of concern
Patients with PUD/GI bleeding history, nasal polyps, impaired liver or renal function, hypertension, CHF, asthma, dehydration or fluid retention and patients over 65 will be cautioned that they have an increased probability of developing adverse reactions to the medication. Patients taking anti-hypertensive medications (diuretics, beta blockers, ACE inhibitors, angiotensin II receptor blockers), methotrexate, fluconazole, lithium or consuming ethanol products will be cautioned that Celecoxib might interfere with the efficacy of these medications.
Adverse reactions
Patients will be followed closely for possible development of adverse reactions to Celecoxib. Most common side effects include dyspepsia, diarrhea, abdominal pain, flatulence, peripheral edema, dizziness, pharyngitis. However, serious adverse reactions can also occur more rarely, including GI ulceration and bleeding, esophagitis, anaphylactoid reactions, angioedema, hypertension, CHF, hepatotoxicity, renal failure, anemia and blood dyscrasias, erythema multiforme, exfoliative dermatitis, Stevens-Johnson syndrome, toxic epidermal necrolysis.
Screening visit
Patients were informed of the nature of the study and signed a consent form. Inclusion/exclusion criteria were evaluated through medical history review, brief periodontal probing and caries evaluation as well as review of any existing intraoral radiographs. Female subjects of reproductive age underwent a pregnancy test and pregnant patients were disqualified. Non pregnant patients were reminded that they will be required to use two forms of proven birth control measures during the first 6 months of the study, as indicated in the consent form. If patients fulfilled the criteria for entering the study, their baseline visit appointment was scheduled. Patients who did not qualify, where referred to the appropriate clinic for further treatment, if necessary.
First Visit (Baseline): Full mouth periodontal examination performed (plaque index, probing depth and clinical attachment level at 6 sites per tooth, bleeding on probing, mobility index). Full-mouth standardized vertical bitewing radiographs were taken. Oral Hygiene Instructions (OHI) were given.
Second visit (1-2 wks): A second pregnancy test was administered to female subjects of reproductive age to minimize the possibility of false negative results in the previous test. Gingival crevicular fluid samples were collected from a single site of 6 representative teeth (based on baseline examination), processed with Periotron 8000, frozen in liquid nitrogen and stored at -80°C for further analysis. Scaling and root planning of the UR and LR quadrants under local anesthesia, performed if required. Patients were randomly assigned to the test (200 mg Celecoxib qd) or control (Placebo) groups and will be provided with three months’ worth of medication or placebo capsules, being instructed to take one capsule per day. Celecoxib and same size/shape/color placebo capsules was provided in identical containers labeled 1-150, assigned randomly to Celecoxib or placebo. Active drug and placebo were distributed under the supervision of the hospital pharmacy. The containers were dispensed consecutively in increasing number order and neither the examiner nor the patient was aware of the true contents of the container. The code was kept by the hospital pharmacy as well as the Chair of the DSMB and was broken only if a patient developed adverse reactions during the study.
Third visit (2-3 wks): Scaling and root planning of the UL and LL quadrants under local anesthesia, if required.
Fourth visit (3 mos): Full mouth periodontal examination, Collection and processing of gingival crevicular fluid samples from the same 6 teeth used in visit one. OHI, Prophylaxis. Patient compliance in taking the prescribed medication will be assessed (patients will be required to return the original medicatio/placebo container). A second container with enough capsules for three more months will be given to each patient.
Fifth visit (6 mos): Full mouth periodontal examination, Collection and processing of gingival crevicular fluid samples from the same 6 teeth used in visit one. Full mouth standardized vertical bitewing radiographs. OHI, Prophylaxis. Patient compliance in taking the prescribed medication will be assessed.
Sixth Visit (9 mos): Full mouth periodontal examination. Collection and processing of gingival crevicular fluid samples from the same 6 teeth used in visit one. OHI, Prophylaxis.
Seventh Visit (12 mos): Full mouth periodontal examination. Collection and processing of gingival crevicular fluid samples from the same 6 teeth used in visit one. Full mouth standardized vertical bitewing radiographs. OHI, Prophylaxis. Completion of study protocol.
Mechanical Treatment: SRP under Local Anesthesia.
Periodontal Support Treatment: 3 months for 12 months.
Primary outcome measures: Probing Depth (PD) and Clinical Attachment Level (CAL), GCF fluid collection with perio-paper strips. At this point the patients were referred to the post-graduate periodontal clinic if surgical periodontal therapy was deemed necessary. Otherwise, they will were advised to enter a 3 month periodontal maintenance schedule at TUSDM or their private dentist/ periodontist. Also, if at any time during the study there was evidence of rapid clinical attachment loss (> 3mm in three months or > 4mm in six months), or any emergency situation developed (pain, abscess), patients were dropped out of the study and referred to the post-graduate periodontal clinic for treatment. Furthermore, patients were dropped out of the study if they developed any adverse reactions that could be attributed to the medication, as discussed above.
PGE2 Elution: Perio strips were eluted into a 1XPBS 0.05% Tween solution at 4o
PGE2 Quantification: The amount of PGE2 present in the lysate was determined by using an enzyme-linked immunosorbent assay (ELISA, Caymen Chemical Ann, Arbor, MI, USA).
Statistical analysis
Sample Size was similar in design to that of Caton JG et al., (Journal of Periodontology 71:521-532, 2000), where six month changes in a placebo group were shown to be: A) Mod: CAL = 0.83 (se = 0.05, n = 92) and PD = 0.68 (se = 0.05, n = 92) B) Sev: CAL = 1.14 (se = 0.13, n = 78) and PD = 1.14 (se = 0.12, n = 78) Based on these numbers a sample size of 60 in each group will be able to detect six month differences between the treatment groups of 0.25 (Mod) and 0.57 (Sev), based on an independent sample t-test at a level of significance of 0.05 and a power of 80%. Change in clinical attachment loss probing depth and PGE2 was analyzed using one-way analysis of variance and spearman rank correlation. These analyses were done separately for the two treatment groups (Celebrex and Placebo) as well as for each time point (baseline to 3 months, baseline to 6 months and 3 to 6 months).
RESULTS
Measurements of PGE2 revealed that there was a modest positive relationship between PD and PGE2 (spearman rank correlation: r = 0.12, p = 0.037) and a significant relationship between CAL and PGE2 (r = 0.028, p = 0.001) (Figure 2).
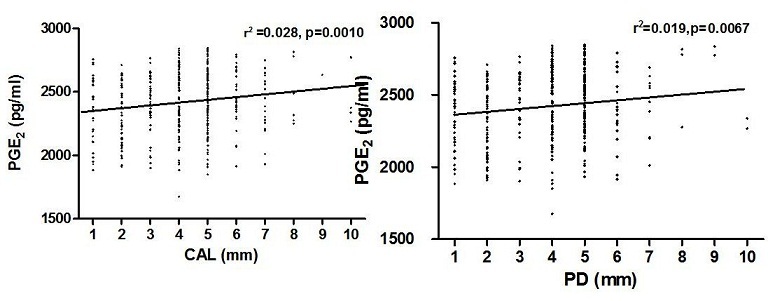 Figure 2: Relationship between PGE2 levels PD and CAL.
Figure 2: Relationship between PGE2 levels PD and CAL.
Clinically PD and CAL improved significantly over the 6-month period post treatment. There was a modest positive relationship between PD and PGE2 (spearman rank correlation: r = 0.12, p = 0.037) (Figure 3).
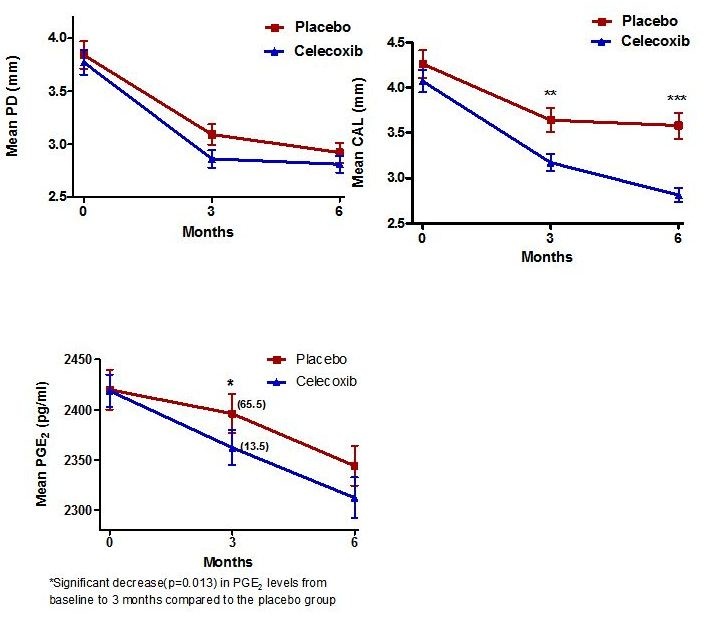 Figure 3: Clinical correlation between PD, CAL, and PGE2 over 6-month period post treatment.
Figure 3: Clinical correlation between PD, CAL, and PGE2 over 6-month period post treatment.
In the Celecoxib group, those who had a decrease in PD from baseline to 6 months showed a statistically significant drop in PGE2 levels, when compared to those who did not decrease their PD (172.8 pg/ml vs. 97.7 pg/ml, p = 0.01).
In addition, the COX-2 group demonstrated a statistically significant decrease in PGE2 levels from baseline to 3 months compared to the placebo group (65.5 pg/ml vs. 13.5 pg/ml, p = 0.013), but not at 6 months (109.8 pg/ml vs. 71.2 pg/ml, p = 0.12) (Figure 4).
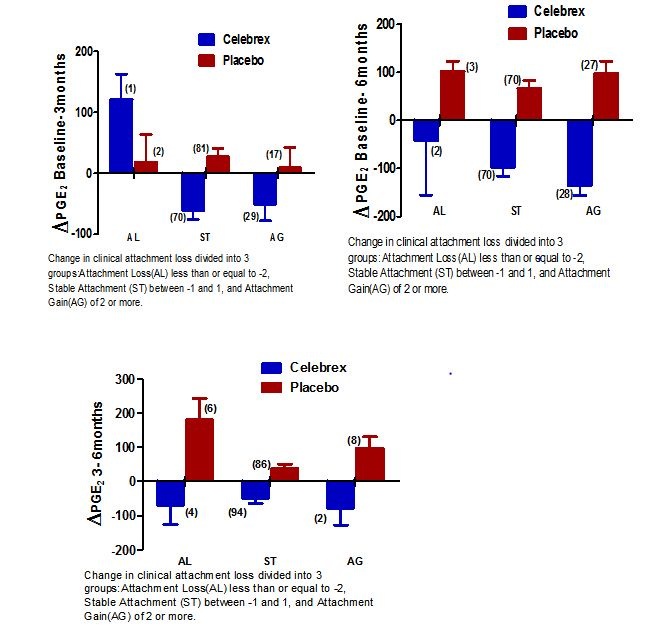 Figure 4: Comparison of PGE2 levels over different times in conjunction to clinical attachment loss.
Figure 4: Comparison of PGE2 levels over different times in conjunction to clinical attachment loss.
Cytokine and stimulating factor levels were also analyzed for 8 samples to screen for potential trends or potential markers of inflammation in the Celecoxib and placebo group as shown in table 1. The only trend observed from the GCF sample analysis is elevated IL-8 values associated with the treatment group.
|
Sample Identification |
IL-1β |
IL-2 |
IL-4 |
IL-5 |
IL-6 |
IL-7 |
IL-8 |
IL-10 |
IL-12p70 |
IL-13 |
IFN-γ |
GM-CSF |
TNF-α |
|
Celecoxib Treatment Group |
< 3 |
< 3 |
11 |
< 3 |
7 |
< 3 |
1,356 |
6 |
< 3 |
< 4 |
< 4 |
12 |
< 3 |
|
3 |
< 3 |
23 |
< 3 |
5 |
< 3 |
1,518 |
4 |
< 3 |
< 4 |
< 4 |
11 |
< 3 |
|
|
8 |
< 3 |
44 |
< 3 |
8 |
< 3 |
2,245 |
< 3 |
< 3 |
< 4 |
5 |
13 |
< 3 |
|
|
4 |
3 |
41 |
< 3 |
9 |
< 3 |
2,939 |
5 |
< 3 |
< 4 |
4 |
15 |
< 3 |
|
|
Placebo Treatment Group |
< 3 |
< 3 |
< 7 |
< 3 |
< 3 |
< 3 |
266 |
< 3 |
< 3 |
< 4 |
< 4 |
< 4 |
< 3 |
|
< 3 |
< 3 |
17 |
< 3 |
< 3 |
< 3 |
295 |
< 3 |
< 3 |
< 4 |
< 4 |
< 4 |
< 3 |
|
|
< 3 |
< 3 |
11 |
< 3 |
< 3 |
< 3 |
77 |
< 3 |
< 3 |
< 4 |
< 4 |
< 4 |
< 3 |
|
|
< 3 |
< 3 |
42 |
< 3 |
< 3 |
< 3 |
192 |
< 3 |
< 3 |
< 4 |
4 |
10 |
< 3 |
Table 1: Data were obtained using Human Cytokine Lincoplex assay. Cytokine units are as follows:
|
IL-1β, pg/ml |
IL-5, pg/ml |
IL-8, pg/ml |
IL-13, pg/ml |
TNF-a, pg/ml |
|
IL-2, pg/ml |
IL-6, pg/ml |
IL-10, pg/ml |
IFN-γ, pg/ml |
|
|
IL-4, pg/ml |
IL-7, pg/ml |
IL-12p70, pg/ml |
GM-CSF, pg/ml |
Finally, no adverse events (as described above) were reported in this study with COX-2 selective inhibitors and no diminished patient pool contributing to the analysis as a result of adverse effects was noted.
DISCUSSION
This study analyzed GCF samples from patients that participated in a placebo controlled, randomized clinical trial, that evaluated the efficacy of Celecoxib (COX-2 inhibitor) in conjunction with Scaling and Root Planning (SRP) in subjects with Chronic Periodontitis (CP) [1]. The Yen et al., J Periodontol 79:104, 2008 study showed PD reduction and CAL gain greater in the Celecoxib group of moderate and deep sites. Celecoxib showed to be an effective adjunctive treatment to SRP to reduce progressive attachment loss in subjects with CP up to 6 months post drug administration. In this study, patients treated with Celecoxib, a specific COX-2 inhibitor, showed significant decrease in PGE2 levels compared with the placebo group at the 3 month follow up but not at 6 months. A modest positive relationship between PD and PGE2 was observed in the study. Decreases in PD from baseline to 6 month in the Celecoxib group were associated with a significant drop in PGE2 levels, when compared to those who did not decrease their PD. Despite the variability in reading, less variability and better correlation between PGE2 levels was associated with the moderate and severe site sites. In addition, GCF samples (n = 8) were screened using a commercially available assay by LINCOplex™ Immunoassay kits (EMD Millipore Corporation, Billerica, MA, USA) for cytokines and stimulating factors (IL-1b, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 p70, IL-13, TNF-a, IFN-g, and GM-CSF) for both placebo and Celecoxib group. Data are not included in this paper but increased levels of IL-8 were seen in the Celecoxib group when compared to the placebo at 3 months. IL-8 is important for PMN recruitment and also promotes monocyte adhesion [17,18]. The finding of higher levels of IL-8 in the GCF of Celecoxib samples is unexpected, as Celecoxib is an anti- inflammatory. However, Mathur et al., demonstrated diminished host response associated with low GCF IL-8 levels [19]. A deficient or ineffective chemokine IL-8 gradient may impair PMN function and increased susceptibility to periodontitis.
Limitation of the present analysis is the low statistical power, inability to sample PMN from the subjects, and lack of information regarding subgingival species. These issues need to be considered in future mediator inflammatory studies as well as in drug efficiency studies. These findings need to be further analyzed in a larger pool of patients.
CONCLUSION
In summary, suggest that Celecoxib (COX-2) in conjunction to SRP significantly reduces PGE2 levels in the GCF in the first 3 months post treatment in CP patients. This decrease is associated with improved clinical outcomes. These findings need to be further analyzed in a larger pool of patients.
ACKNOWLEDGMENT
This study was an investigator-initiated study funded by Pfizer.
REFERENCES
- Yen CA, Damoulis PD, Stark PC, Hibberd PL, Singh M, et al. (2008) The effect of a selective cyclooxygenase-2 inhibitor (Celecoxib) on chronic periodontitis. J Periodontol 79: 104-113.
- Offenbacher S, Barros SP, Beck JD (2008) Rethinking periodontal inflammation. J periodontology 79: 1577-1584.
- Alpagot T, Konopka K, Bhattacharyya M, Gebremedhin S, Duzgunes N (2008) The association between gingival crevicular fluid TGF-beta1 levels and periodontal status in HIV-1(+) patients. J Periodontol 79: 123-130.
- Vane J, Botting R (1987) Inflammation and the mechanism of action of anti-inflammatory drugs. FASEB J 1: 89-96.
- Gustafson GT, Lerner U (1984) Bradykinin stimulates bone resorption and lysosomal-enzyme release in cultured mouse calvaria. Biochem J 219: 329-332.
- Subongkot S, Frame D, Leslie W, Drajer D (2003) Selective cyclooxygenase-2 inhibition: a target in cancer prevention and treatment. Pharmacotherapy 23: 9-28.
- elAttar TM, Lin HS, Killoy WJ, Vanderhoek JY, Goodson JM (1986) Hydroxy fatty acids and prostaglandin formation in diseased human periodontal pocket tissue. J Periodontal Res 21: 169-176.
- Offenbacher S, Odle BM, Van Dyke TE (1986) The use of crevicular fluid prostaglandin E2 levels as a predictor of periodontal attachment loss. J Periodontal Res 21: 101-112.
- Klein DC, Raisz LG (1970) Prostaglandins: stimulation of bone resorption in tissue culture. Endocrinology 86: 1436-1440.
- Offenbacher S, Farr DH, Goodson JM (1981) Measurement of prostaglandin E in crevicular fluid. J Clin Periodontol 8: 359-367.
- Offenbacher S, Odle BM, Gray RC, Van Dyke TE (1984) Crevicular fluid prostaglandin E levels as a measure of the periodontal disease status of adult and juvenile periodontitis patients. J Periodontal Res 19: 1-13.
- Williams RC, Jeffcoat MK, Howell TH, Reddy MS, Johnson HG, et al. (1988) Ibuprofen: an inhibitor of alveolar bone resorption in beagles. J Periodontal Res 23: 225-229.
- Williams RC, Jeffcoat MK, Howell TH, Rolla A, Stubbs D, et al. (1989) Altering the progression of human alveolar bone loss with the non-steroidal anti-inflammatory drug flurbiprofen. J Periodontol 60: 485-490.
- O'Brien TP, Roszkowski MT, Wolff LF, Hinrichs JE, Hargreaves KM (1996) Effect of a non-steroidal anti-inflammatory drug on tissue levels of immunoreactive prostaglandin E2, immunoreactive leukotriene, and pain after periodontal surgery. J Periodontol 67: 1307-1316.
- Reddy MS, Geurs NC, Gunsolley JC (2003) Periodontal host modulation with antiproteinase, anti-inflammatory, and bone-sparing agents. A systematic review. Ann Periodontol 8: 12-37.
- Ryan ME (2002) Clinical applications for host modulatory therapy. Compend Contin Educ Dent 23: 1071-1076.
- Tonetti MS (1997) Molecular factors associated with compartmentalization of gingival immune responses and transepithelial neutrophil migration. J Periodontal Res 32: 104-109.
- Tonetti MS, Imboden MA, Lang NP (1998) Neutrophil migration into the gingival sulcus is associated with transepithelial gradients of interleukin-8 and ICAM-1. J Periodontol 69: 1139-1147.
- Mathur A, Michalowicz B, Castillo M, Aeppli D (1996) Interleukin-1 alpha, interleukin-8 and interferon-alpha levels in gingival crevicular fluid. J Periodontal Res 31: 489-495.
Citation: Panos IA, Damoulis PD, Stark PC, Papas AS (2020) Celecoxib Decreases Gcf Prostaglandin E2 Levels in Chronic Periodontitis Patients. J Dent Oral Health Cosmesis 5: 012.
Copyright: © 2020 Iris Anastasia Panos, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

