
Different Applications of Stem Cells Therapy for Degenerative Retinal Diseases
*Corresponding Author(s):
Antonio FloridoUniversity La Sapienza Of Rome, Polo Pontino - Ospedale A. Fiorini Di Terracina, Italy
Tel:+39 3486500312,
Email:antonioflorido@hotmail.it
Abstract
Retinal degenerative diseases, such as Stargardt’s Disease (SD), glaucoma, Retinitis Pigmentosa (RP), Age-Related Macular Degeneration (AMD) or Diabetic Retinopathy (DR), represent the main causes of a decreased quality of vision and blindness worldwide. The progression and management of these conditions have always represented a challenge, but promising new evidences about the efficacy of Mesenchymal Stem Cells (MSCs) as therapy for these diseases has been shown. The therapeutic potential of MSCs lies on its ability to release paracrine factors with neuroprotective, immunomodulatory and anti-angiogenic properties that stimulate the Retinal Pigmented Epithelium (RPE) and are even similar to those produced by RPE. In literature we can find many studies conducted animal models, in which MSCs proved their efficacy in stopping the progression of retinal degeneration and for rescuing photoreceptors in the dormant phase. Furthermore, they retain a differentiation potential which allow them to differentiate into various cell types, including the cells of the retina. By all of those properties it is clear how MSCs result an important therapy option in these pathologies. In this review we summarize the various properties of MSCs and their promising applications in various retinal diseases, enhanching a new clinical approach on pathologies which otherwise have a difficult managing and a unfavorable prognosis.
Keywords
Cell therapy; Mesenchymal stem cells; Retinal degenerative diseases; Retinitis pigmentosa
Introduction
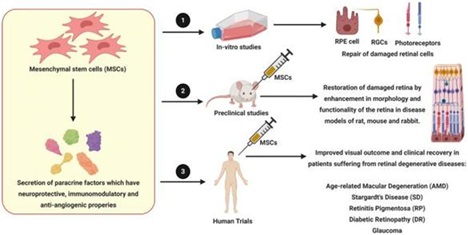 Application of MSCs [1]
Application of MSCs [1]
The retina is the innermost layer of the eye and represents its nervous tunic. It consists of ten layers, which are composed of different type of cells which permits the capture and transduction of light stimuli in electrical impulses, then delivered to the visual cortex of the brain. All retinal layers are connected and work together in order to permit the vision, therefore a damage to any of their structure can result in visual impairment. This can derive from a wide range of retinal degenerative disease. While the etiology and pathogenesis of this diseases are different and some still not well known, they share many retinal damage factors, such as ROS, vascular defects, overproduction of pro-inflammatory cytokines, defects of blood-retinal barrier and aging [2-4].
In this review we considered some of the most common retinal degenerative diseases (RDDs), such as age-related macular degeneration (AMD), retinitis pigmentosa (RP), glaucoma and Stargardt’s disease (SD). These diseases have in common the damage of retinal cells, whether they be the ganglion cells, photoreceptors or retinal pigment epithelium (RPE) cells. At the same time, they are responsible of neuro-inflammations, microglial activation, angiogenesis and retinal gliosis.
To date, there’s not a proper and effective treatment for RDDs, since retina doesn’t have a regenerative ability. Therefore, new approaches that aim to slow vision loss and/or restore vision are actually object of study, such as gene therapy, neuroprotection, new anti-VEGF molecules (vascular endothelium growth factors), and stem cell therapy [5].
Mesenchymal stem cells (MSCs) have been isolated from different tissue sources like bone marrow (bone marrow-derived MSC, BM-MSC), adipose tissue(adipose-mesenchymal derived stem cells, ASC), dental pulp (dental pulp stem cells, DPSC), umbilical cord blood (umbilical cord-derived MSCs, UC-MSC), Wharton's jelly MSCs (WJ-MSCs), amniotic membrane. Several studies in literature analyzed the potential regenerative ability of MSCs of retinal cells for therapeutical applications in several retinal degenerative disorders [6]. There are many reasons for considering MSCs as a possible treatment option. Firstly, they can produce paracrine signaling through secretion of neurotropic factors, which provide a reparative effect on neuro-retinal cells. Secondly, MSCs have shown immunomodulatory properties that can module the pro-inflammatory microenvironment, which is usually present in these diseases. Thirdly, MSCs are capable to secrete anti-angiogenic factors, which counter the the pro-angiogenesis mediators, crucial in the pathogenesis of DR and wet AMD for example [7].
Even though cell-based therapy can be considered as a promising opportunity in these retinal pathological conditions, it is important to report how several patients treated with intraocular injection of autologous MSCs derived from bone marrow/adipose tissue encountered loss of vision after intraocular injection of autologous MSCs derived from bone marrow/adipose tissue [8,9]. A probable cause of these outcomes may be the trans-differentiation of injected MSCs into myofibroblast-like cells, which can induce a proliferative vitreoretinopathy (PVR) which can lead retinal detachment.
Given these possible complications, it is crucial to understand better the interaction be between administered MSCs and the degenerative retinal environment of the patient. There’s still the a need of evidence about the safety and efficacy of stem/progenitor cell-based transplantation therapy, but there are several promising result to date [10].
Table 1 shows the summary of ongoing and concluded clinical trials 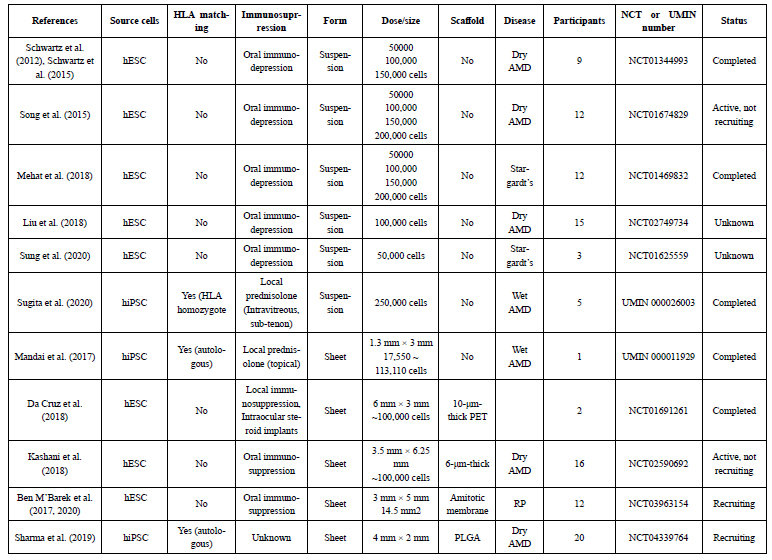 Table 1: Summary of ongoing and concluded clinical trials of stem-cell-derived RPE transplantation [11].
Table 1: Summary of ongoing and concluded clinical trials of stem-cell-derived RPE transplantation [11].
NOTE: Studies identified based on a search in PubMed NIH at the end of October 2020.
Abbreviations: AMD: Age-Related Macular Degeneration; CPCB-RPE: California Project to Cure Blindness–Retinal Pigment Epithelium; PET: Polyethylene Terephthalate; PLGA: Poly Lactic-Co-Glycolic Acid; RP: Retinitis Pigmentosa.
Possible Roles Of Mscs In Treatment Of Retinal Diseases
Until date, these retinal diseases have no curative treatment. The main goal of stem cell therapy is to slow down the tissue degeneration caused by the underlying pathology. By delivering ESCs, MSCs and iPSCs into the eye, it is possible to exert a renewal effect on the damaged retinal tissue. Moreover, they dampen the pro-inflammatory factors which contribute to retinal cells death [12]. In particular, MSCs have been gathered from several tissue sources such as bone marrow, adipose tissue, dental pulp, umbilical cord blood, amniotic membrane and they are considered in many studies among the best candidates for a therapy regimen in RDDs. The reason for this is that they secrete paracrine factors, exosomes and mitochondria into host cells. As follow we resume some of the key points of these features:
Paracrine neuroprotective factors
Bone marrow derived mesenchymal stem cells (BMSCs) are capable to produce and secrete a wide range of neurotrophic factors (NTFs) such as ciliary neurotrophic factor (CNTF), BDNF, glial cell derived neurotrophic factor (GDNF), platelet derived growth factor (PDGF), nerve growth factor (NGF), neurotrophin-3, 4/5 (NT-3, 4/5), insulin-like growth factor 1 (IGF1), basic Fibroblast growth factor (FGF2), PEDF and erythropoietin (EPO). In particular, these neurotrophic factors find their receptors on retina cells and enhance their differentiation, neural cell survival, axonal outgrowth, neural cell attachment, while stopping neural cell apoptosis. The signaling pathways activated by the NTFs, such as P13K/AKT, P13K/IAP, PLC/IP3/PKC, MAPK/ERK and JAK/STAT3 have neuroprotective effect on the neuro-retinal cells. In many studies it has been shown how the beneficial effects of these mediators. The neuroprotective role was demonstrated in a study by Cui et al conducted in in-vivo, where co-culturing BMSCs with retinal ganglion cells (RGCs) reduced hydrogen peroxide (H2O2) induced injury in RGCs through the expression of neurotrophins, BDNF, CNTF and reduced the expression of pro-inflammatory factors interleukin 1β(IL1β) and tumor necrosis factor α(TNFα) by RGCs [13]. In another work, Osborne et al and Johnson et al showed how PDGF secreted by BMSCs can exert a protection on RGCs in both an ex vivo and preclinical models [14]. Mead et al stated that NGF, BDNF and NT-3 secreted by BMSCs have protective effects on RGCs; moreover, they showed that this effect is lost when tropomyosin related kinase (TrK) and PDGF receptor α(PDGFRα) get inhibited in RGCs [15]. Intravitreal transplantation of BMSCs, which were able to secrete GDNF and BDNF, was associated with an higher number of RGCs compared to the control group in an experimental optic nerve crush model. In the same way, it was possible to observ long-term neuroprotection and axon regeneration of RGCs after transplantation of BMSCs, which was attributed to an increased expression of FGF2 and IL1β in the RGC layer that activated the PI3/AKT signaling cascade and rescued RGCs. In a study of Martin et al., it was observed a significant increase in neuroprotective (Dll4, Crim-1, Glupican-3, Cntn1), anti-inflammatory (Transforming Growth Factor β and IL10, 13, 11, 4) molecules as well as proteins associated with anti-oxidant (haptoglobin), anti-apoptotic (Apex1) activity and protein homeostasis (Hsp10, Hsp60, Hsp70, Hsp20, Hsp27, Kctd10, Pyk2, clusterin) in the secretome of human BMSCs co-cultured with neuroretinal explants [16].
MSCs derived extracellular vescicles (MSC-EVs)
MSC-EVs or exosomes are secreted, bilipid layered, nano dimensional micro vesicles. These structures can encapsulate functional molecules, like proteins, lipids, miRNAs which are able to exert important therapeutic effects. These vescicles were observed to be endocytosed by retinal neurons, microglia and RGCs via caveolar mediated endocytic pathway, facilitated by heparin sulfate proteoglycans (HSPGs). Furthermore, their interaction with proteins of the vitreous humor resulted in a prolonged retention of EVs in the eye. Yu and co-workers observed how the administration of MSC-EVs in vitreous was equally efficient when compared with transplanted MSCs, not only in reducing apoptosis and damage, but even in augmenting vision in an experimental model of retinal laser injury. Additionally, MSC-EVs dampened retinal damage processes through the downregulation of pro-inflammatory mediators, intercellular adhesion molecule 1 (ICAM1), monocyte chemoattractant protein 1 (MCP1), VEGF-A and TNFα [17].
MSCs weaken inflammatory responses
The so called ocular immune privilege is the capability of the eye to restrain intraocular infiammation, so that there’s a protective effect on a the visual components from damage and therefore preserving visual acuity. This is an articulated phenomenon made possible by the blood-retinal barrier (BRB) which efficiently separates the eye from the immune system along with local inhibition of both adaptive and innate immune responses by the ocular microenvironment, and ocular-specific mechanisms cause systemic activation of immunosuppressive regulatory T cells. Nevertheless, in every retinal degenerative diseases such as RP, AMD, glaucoma and DR, there’s an abundance of proinflammatory cytokines associated with an infiltration of immune cells leading to breakage of the BRB.
Many studies in literature highlighted how intravitreal and periorbital administration of BMSCs granted a significant reduction of inflammatory cytokines in the retinal microenvironment, infiltration of macrophages and CD4+ T cells [18].
MSCs regulate angiogenesis
Pathological retinal angiogenesis, leads to derangement and produce aberrant blood vessels that compromise the retinal tissue organization. These neovascularizations break through the outer retina and the macular pit, which is physiologically an avascular zone. The disruption of macular structure compromises the normal vision. Many retinal pathological conditions lead to aberrant angiogenesis with progressive loss of vision. Basically, every time there are low levels of oxygen, tissues may respond with aberrant angiogenesis. Therefore, this condition can be observed in many pathological pictures.
Kim et al., observed how the administration of intraperitoneal injection of human placental amniotic membrane derived MSCs (AMSCs) in a mouse model of oxygen induced retinopathy resulted in significant reduction of neovascularization through TGFβ1 expression, which was in fact blocked when AMSCs were transfected with TGFβ1 siRNA [19]. Ghazaryan et al., showed that sub-conjunctival injection of BMSCs had an adjuvant effect on corneal wound healing and significantly reduced the neovascularization by downregulating VEGF and matrix metalloproteinase-9 (MMP-9) expression [20]. Through the use of ADSCs intravitreal injectons of eyes of a diabetic mouse model, although the intraocular levels of VEGF and PDGF was unaffected, the expression levels of TSP1 increased significantly [13]. TSP1, primarily produced by RPE, choroid and mÜller glial cells in the healthy eye prevents VEGF receptor 2 (VEGFR2) activation by disrupting the receptor’s association with CD47 and terminates the VEGF signaling to AKT- endothelial nitric oxide synthase pathway [14,15]. TSP1 also binds to CD36 and recruits Src homology 2 domain- containing protein tyrosine phosphatase (SHP1) to the CD36-VEGFR2 complex in the microvascular endothelial cells, which in turn dephosphorylates VEGFR2 and inhibits angiogenesis [16]. It has been proved how the successful reconstruction of damaged ocular tissues by MSCs relies more on the release of paracrine anti-inflammatory and anti-angiogenic factors than in the differentiation into ocular cells. Consequently, when human BMSCs were intravitreally implanted in an oxygen induced retinopathy mouse model, it significantly reduced retinal neovascularization.
MSCs donate mitochondria
It has been reported in several studies how MSCs are able to transfer healthy, functional mitochondria via gap junctions, tunneling nanotubes (TNTs) [17] and exosomes to the damaged cells for its regeneration [21]. Furthermore it has been demonstrated the enhancement of mitochondrial bioenergetics by MSCs in the injured cells in spinal cord [21], bronchial epithelia [22], corneal epithelia [23], cardiomyocytes [24-26] and cells affected by neurotoxicity [27].
Even though MSCs do not pass-through inner limiting membrane (ILM) of the retina when injected intravitreally, the mitochondria donated efficiently permeate the ILM, limiting the RGC death.
MSCs differentiate into retinal cells
Bone marrow derived mesenchymal stem cells (BMSCs), smniotic membrane derived mesenchymal stem cells (AMSCs), umbilical cord blood derived mesenchymal stem cells (UMSCs) and dental pulp derived mesenchymal stem cells (DPSCs) showed promising effect about the differentiation of these cells into various cells of retinal tissue in vitro, even expressing their genes. It has been already tested in some studies the functionality in vitro of the differentiated cells, however, there’s the need of further preclinical studies in order to understand the safety, immunogenicity and function of the transplanted cells in vivo.
Therapeutical Applications Of Mscs In The Treatment Of Retinal Degenerative Diseases
Retinal degenerative diseases can be considered as a wide group of disorders which can lead to blindness, including age-related macular degeneration (AMD), retinitis pigmentosa (RP), diabetic retinopathy (DR), pediatric Stargardt’s disease (SD), glaucoma and other diseases. These pathologies have a multifactorial etiology, but they share the fact that they lead to visual impairment and lately to blindness. Moreover, they have in common the damage and death of retinal cells and the degeneration of photoreceptors [1,28]. In AMD the degeneration of RPE and Bruch’s membrane leads to the loss of photoreceptors. RP is characterized by the disruption of the rod photoreceptors at first, then of the cones, due to a genetic defect. In DR, since early stages, a damage to pericytes, endothelial cells and retina’s neuronal cells is observed. SD is an hereditary disease where the loss of RPE and photoreceptors brings to loss of vision in young patients. In glaucoma, an high intraocular pressure (IOP) causes a progressive damage to retinal ganglion cells (RGCs) [29,30]. The treatment currently available for these diseases is limited, in fact it is mainly directed to the slowing of the disease progression. Late stages of RD can only benefit from laser photocoagulation and intraocular injection of inhibitors of vascular endothelial growth factors (VEGF), the latter used also in wet AMD. In some cases, the treatment is surgery, such as vitrectomy or microsurgery strategies, which are much more invasive and can lead to risky complications. On the other hand, stem cells could represent a novel approach to these pathologies, due to their potential to regenerate the damaged retinal tissue, as happens in these age-related retinal disorders. There are already many studies on animals demonstrating the therapeutical potential of MSCs on degenerative retinal diseases [31-33]. In fact, evidences proved how these cells can provide a regeneration of many retinal cells, such as RPE cells, photoreceptors and axons, and also augment the survival of RGCs [34,35].
Use of MSCs in age-related macular degeneration
Age-related macular degeneration is listed as a leading cause of blindness in the elderly population; in particular, people over 70 years of age are at highest risk [36]. The vision loss is due to the disruption of RPE, without which the photoreceptors loose its nutrition and homeostasis’ maintenance function, causing their progressive death. The pathogenesis of AMD derives from many genetic and environmental factors [37], which leads in early stages to an accumulation of amorphous deposits under the RPE, visible as drusen during fundoscopy exam or optical coherence tomography (OCT). These deposits can trigger inflammation, which pushes towards degeneration of retinal cells [2]. Late stages of the disease include two different forms, dry AMD, called also geographic atrophy, and wet AMD, which consist in a choroidal neovascularization. Dry AMD is a result of the degradation of RPE and Bruch’s membrane, with consequential loss of photoreceptors. On the other hand, the accumulation of materials between RPE and Bruch’s membrane can results in its detachment leading to wet AMD. The consequent neovascularization is characterized by abnormal and leaky capillaries, which causes the accumulation of subretinal fluid or macular hemorrhages and thus the central vision loss [38]. Currently available therapies are effective only on wet AMD, while geographic atrophy, which affects most of the AMD population, remains incurable. Wet AMD patients can benefit from anti-VEGF or steroids intraocular injections, retinal photodynamic and laser photocoagulation therapy in order to alleviate neovascularization [39,40]. In studies on animals, where AMD disease was artificially provoked by using sodium iodate (NaIO3), which normally cause degeneration of RPE and photoreceptors, MSCs implantation granted protection of these retinal cells from NaIO3 [41]. Recently, some studies have also been conducted on humans. In 2012, Schwartz et al. provided preliminary results about human ESCs subretinal transplantation in patients suffering from AMD and SD, showing the safety of the procedure on the patient’s eyes in the next 4 months. After a 22 month follow up, which recruited 9 dry AMD and 9 SD patient’s eyes, a benefit in term of BCVA was observed in 10 patients, while it remained stable in 7 eyes and decreased in 1 eye. Untreated eyes showed no improvement in BCVA [42,43]. In a work of Kumar and colleagues bone marrow MSCs were administrated through intravitreal injections in 60 advanced dry AMD patients. After a 6-month follow up, authors observed an improvement BCVA, as well as electrophysiological and anatomical amelioration [44]. In a study of Limoli et al., authors used the Limoli Retinal Restoration Technique on 36 eyes of 25 patients suffering from dry AMD. The technique consisted in surgical implantation in the suprachoroidal space of autologous cells, platelets derived from platelet-rich plasma and adipose derived MSCs. After 6 months, treated patients showed better BCVA compared to baseline values, expecially in patients with higher retinal thickness [45,46].
Use of MSCs in retinitis pigmentosa
Retinitis Pigmentosa is a hereditary degenerative retinal disease which represent one of the main causes of vision loss below middle age population, with a world prevalence of 1:4000 [47]. It may occur with autosomal recessive (AR), autosomal dominant (AD) or X-linked inheritance patterns. Although it involves only the retina, RP can present also as part of a syndrome; among these, Usher’s syndrome is the most common one [48]. At first, patients suffer from loss of night vision (nyctalopia) and limitation of pheriperal vision, due to the disruption of the rod photoreceptors caused by gene mutations, then in later stages it hits cones, causing the loss of central vision and colours discrimination [49]. To date, RP can be caused by almost 3000 mutations of almost 200 genes [50]. RP pathogenesis takes place from its characteristic gene’s mutations, affecting rods in peripheral retina. As a result, the disrupted rods have a lower demand in term of metabolic processes. At the same time, the availability of a rich blood supply and high metabolic rate could lead to oxidative stress affecting cone’s function. Moreover, in RP there’s an increased expression of proinflammatory cytokines secondary to extrabulbar inflammatory processes, which contributes to the cone’s disfunction [51,52]. From 20 to 30% of the AD cases is caused by rhodopsine gene (RHO), while Usherin (USH2A) is the most common gene involved in AR form (15% of cases), and the X-linked one is due to the retinitis pigmentosa GTPase regulator (RPGR, 70 to 90% of cases) [53]. Treatment of RP is currently very limited, basing mostly on support therapy. According to some evidence, antioxidant vitamins could slow disease’s progression, such as vitamin A and b-carotene [54,55]. The first example of gene therapy for autosomal dominant RP is Luxturna, which is an adeno-associated viral vector containing the complementary DNA of RPE65, whose mutation may cause recessive RP [56]. Another therapeutical approach consists in a transplantation of an artificial retina, which can grant a visual recovery in advanced stage of the disease [57,58]. Regarding stem cells therapy for RP, the aim is to obtain differentiated photoreceptors cells starting from in vitro stem cells, then transferring them in the eye through subretinal implants [59]. Some researchers succeeded in this challenge and showed how these photoreceptors can integrate in the retina of animal models of retinal degeneration diseases, improving visual function [60,61].
In a study of Weiss and Levy, authors administrated autologous bone-marrow stem cells to 33 eyes of 17 patients through retrobulbar, subtenons, intravitreal or intravenous injections, observing an improvement of 7.9 Snellen lines in 15 eyes [62]. Wiacek et al., performed intravitreal injections of autologous bone marrow stem cells in RP patients with a disease story of a few years or more than 10 years, which resulted in a significative improvement in BCVA and best-corrected distance visual acuity (BCDVA) in a 12 months follow-up [63]. In a non-randomized phase I clinical trial involving 14 patients with RP, an intravitreal injection of bone marrow MSCs was performed, aiming to compare the BCVA at 1 and 7 years from the therapy. An increase in BCVA was obtained by every patient after a few months, but it went back to baseline at 1 year [64].
Use of MSCs in DR
Diabetic retinopathy is a diabetes complication which is considered as a multifactorial microvascular disease. It represent a major cause of vision loss in middle age and elderly population. A poorly controlled diabetes is characterized by chronic hyperglycemia, which causes a profound disregulation of metabolism, such as the augmented production of reactive oxygen species, advanced glycation end-products, inflammatory factors and VEGF [65,66]. DR can be classified in two stages: non-proliferative DR (NPDR) and proliferative DR (PDR). In early non-proliferative stages, a progressive damage of microvasculature takes place, with the loss of pericytes and endothelial cells, and there’s also inflammation and degradation of retinal neuron cells [67]. If there’s a progression of the disease in proliferative DR, a macular edema can develop, resulting in vision loss, and local ischemia can lead to proliferation of abnormal new vessels in the retina and in the eye, causing hemorrages [68]. In DR treatment, since the early stages, a precise control of glucose levels and blood pressure is needed [69]. In late stages, in order to slow the progression of the disease, treatment can include retinal laser therapy, intravitreal corticosteroids and anti-VEGF. Steam cells therapies have been hypothesized as a valid solution for RD, considering the retinal cell loss during the progression of the disease. Among MSCs, ASC shown an efficacy when implanted in rat eyes with early-stages of DR, as they improve retinal function [70], and they act as pericytes, so that they could exert a repair of retinal blood vessels [71]. Moreover, they demonstrated the ability of reducing oxidative damage and promote the secretion of neurotrophic factors [72]. In a study of Gu et. al, 10 patients with severe NPDR and 7 with PDR received intravenous administration of autologous BM MSCs. After the 6 months from the therapy, NPDR patients showed a significant improve in BCVA and reduction of macular thickness, while PDR patients had only benefit of slight effects [73].
Use of MSCs in stargardt disease
Stargardt’s disease is an hereditary blinding disease characterized by macular degeneration, due to deposition of lipofuscin-like substance in RPE, leading to photoreceptors cell death. It occurs below the first two decades of life [74,75]. The most common gene involved in this disease is ABCR gene, which disfunction causes the accumulation of lipofuscin molecule components in RPE [76,77]. To date, there is no valid therapeutical approach available to cure or slow the progression of SD. Therefore, the use of stem cell theraphy in order to restore damaged retinal photoreceptors could represent a valid strategy for the disease. Song et al. showed how subretinal transplantation of human ESC-derived cells of RPE were well tolerated in 2 asian patients with SD, showing no adverse effect. Moreover, there was a significative visual acuity improvement during the follow-up period [78]. We already mentioned the work of Schwartz et al., where authors demonstrated the safety and efficacy in terms of BCVA recovery through human ESCs subretinal transplantation in patient’s eyes [44,45].
Use of MSCs in glaucoma
In glaucoma, as like as other retinal degenerative disease, there’s a progressive death of retinal cells, in particular RGCs, due to high values of IOP. This result in the disruption of optic nerve head and consequential vision loss, starting as a reduction in peripheral vision [79,80]. Moreover, there are several other actors in retinal ganglion cell’s degeneration, such as hypoxia, ischemic insults, neuroinflammation, rise in levels of ROS in RGC layer and reduction of neurotrophic factors, occurring as a consequence of high IOP and neuronal damage [79]. Current therapy is based on IOP reduction, through medical treatment involving pharmacological agents, or by using different surgical techniques, ranging from trabeculectomy and drainage implants to MIGS and non-penetrating glaucoma treatments [80]. More recent approaches involved gene therapy, studied to both reduce IOP and increasing of secretion of neurotropic factors [81]. In recent years the attention has also moved towards mesenchymal stem cells transplantation. In a study on rats with glaucoma, Hu et al. showed how BM MSCs can provide a neuroprotective effect, through which they can prevent RGCs damage and degeneration [82]. Limoli et. al conducted a study on 35 eyes of 25 patients suffering from glaucomatous optic neuropathy, where a control group included 21 eyes, while the LRRT group included 14 eyes, which were treated with suprachoroidal autograft of mesenchymal stem cells using Limoli Retinal Restoration Technique. Results after 6 months follow-up showed a significant increase in visual function, regarding BCVA, sensitivity and also close-up visus [83].
Conclusion
Therapeutical application of stem cells, firstly observed and confirmed on animal models, has opened new perspective of treatment of degenerative retinal disease. In fact, while most of the therapeutical options since now avaible has the power of slowing the progression of the disease, stem cells could provide a regeneration of the degenerated cells in the eye. In particular, MSCs appear to exert their therapeutic mechanism through a paracrine effect, so that they could be used to sustain retinal neuron’s function and to stimulate glia to provide a neural repair effect. Thanks to their non-invasive, non-tumorigenic, immunosuppressive and cells supporting effects, MSCs could represent a valid innovative therapy for eye diseases, expecially the ones which currently doesn’t have a proper cure.
References
- Adak S, Magdalene D, Deshmukh S, Das D, Jaganathan BG (2021) A Review on Mesenchymal Stem Cells for Treatment of Retinal Diseases. Stem Cell Rev Rep 17: 1154-1173.
- Shaw PX, Stiles T, Douglas C, Ho D, Fan W, et al. (2016) Oxidative stress, innate immunity, and age-related macular degeneration. AIMS Mol Sci 3: 196-221.
- Semeraro F, Cancarini A, dell'Omo R, Rezzola S, Romano MR, et al. (2015) Diabetic Retinopathy: Vascular and Inflammatory Disease. J Diabetes Res 2015: 582060.
- van Norren D, Vos JJ (2016) Light damage to the retina: an historical approach. Eye (Lond) 30: 169-172.
- Dalkara D, Goureau O, Marazova K, Sahel JA (2016) Let There Be Light: Gene and Cell Therapy for Blindness. Hum Gene Ther 27: 134-147.
- Nancarrow-Lei R, Mafi P, Mafi R, Khan W (2017) A Systemic Review of Adult Mesenchymal Stem Cell Sources and their Multilineage Differentiation Potential Relevant to Musculoskeletal Tissue Repair and Regeneration. Curr Stem Cell Res Ther 12: 601-610.
- Ding SLS, Kumar S, Mok PL (2017) Cellular Reparative Mechanisms of Mesenchymal Stem Cells for Retinal Diseases. Int J Mol Sci 18: 1406.
- Leung EH, Flynn HW Jr, Albini TA, Medina CA (2016) "Retinal Detachment After Subretinal Stem Cell Transplantation. Ophthalmic Surg Lasers Imaging Retina 47: 600-601.
- Kuriyan AE, Albini TA, Townsend JH, Rodriguez M, Pandya HK, et al. (2017) Vision Loss after Intravitreal Injection of Autologous “Stem Cells” for AMD. N Engl J Med 376: 1047-1053.
- Florido A, Vingolo EM, Limoli P, Contento L (2021) Mesenchymal Stem Cells for Treatment of Retinitis Pigmentosa: Short Review. J Stem Cell Res Dev Ther 7: 066.
- Uyama H, Mandai M, Takahashi M (2021) Stem-cell-based therapies for retinal degenerative diseases: Current challenges in the establishment of new treatment strategies. Dev Growth Differ 63: 59-71.
- Limoli PG, Vingolo EM, Limoli C, Nebbioso M (2019) Stem Cell Surgery and Growth Factors in Retinitis Pigmentosa Patients: Pilot Study after Literature Review. Biomedicines 7: 94.
- Ezquer M, Urzua CA, Montecino S, Leal K, Conget P, et al. (2016) Intravitreal administration of multipotent mesenchymal stromal cells triggers a cytoprotective microenvironment in the retina of diabetic mice. Stem Cell Res Ther 7: 42.
- Bazzazi H, Zhang Y, Jafarnejad M, Isenberg JS, Annex BH, et al. (2018) Computer Simulation of TSP1 Inhibition of VEGF–Akt–eNOS: An Angiogenesis Triple Threat. Front Physiol 9: 644.
- Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, et al. (2010) Thrombospondin-1 Inhibits VEGF Receptor-2 Signaling by Disrupting Its Association with CD47. J Biol Chem 285: 38923-38932.
- Chu LY, Ramakrishnan DP, Silverstein RL (2013) Thrombospondin-1 modulates VEGF signaling via CD36 by recruiting SHP-1 to VEGFR2 complex in microvascular endothelial cells. Blood 122: 1822-1832.
- Feng Y, Zhu R, Shen J, Wu J, Lu W, et al. (2019) Human Bone Marrow Mesenchymal Stem Cells Rescue Endothelial Cells Experiencing Chemotherapy Stress by Mitochondrial Transfer Via Tunneling Nanotubes. Stem Cells Dev 28: 674-682.
- Sinclair KA, Yerkovich ST, Hopkins PM, Chambers DC (2016) Characterization of intercellular communication and mitochondrial donation by mesenchymal stromal cells derived from the human lung. Stem Cell Res Ther 7: 91.
- Monsel A, Zhu YG, Gennai S, Hao Q, Hu S, et al. (2015) Therapeutic Effects of Human Mesenchymal Stem Cell–derived Microvesicles in Severe Pneumonia in Mice. Am J Respir Crit Care Med 192: 324-336.
- Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, et al. (2015) Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun 6: 8472.
- Li H, Wang C, He T, Zhao T, Chen YY, et al. (2019) Mitochondrial Transfer from Bone Marrow Mesenchymal Stem Cells to Motor Neurons in Spinal Cord Injury Rats via Gap Junction. Theranostics 9: 2017-2035.
- Li C, Cheung MKH, Han S, Zhang Z, Chen L, et al. (2019) Mesenchymal stem cells and their mitochondrial transfer: a double-edged sword. Biosci Rep 39: BSR20182417.
- Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, et al. (2013) Miro 1 Knockdown in Stem Cells Inhibits Mitochondrial Donation Mediated Rescue of Bronchial Epithelial Injury. EMBO J 33: 994-1010.
- Pacak CA, Preble JM, Kondo H, Seibel P, Levitsky S, et al. Actin-dependent mitochondrial internalization in cardiomyocytes: evidence for rescue of mitochondrial function. Biol Open 4: 622-626.
- Plotnikov EY, Khryapenkova TG, Vasileva AK, Marey MV, Galkina SI, et al. (2008) Cell-to-cell cross-talk between mesenchymal stem cells and cardiomyocytes in co-culture. J Cell Mol Med 12: 1622-1631.
- Jiang D, Gao F, Zhang Y, Wong DS, Li Q, et al. (2016) Mitochondrial transfer of mesenchymal stem cells effectively protects corneal epithelial cells from mitochondrial damage. Cell Death Dis 7: e2467.
- Babenko VA, Silachev DN, Popkov VA, Zorova LD, Pevzner IB, et al. (2018) Miro1 Enhances Mitochondria Transfer from Multipotent Mesenchymal Stem Cells (MMSC) to Neural Cells and Improves the Efficacy of Cell Recovery. Molecules 23: 687.
- Lin TC, Hsu CC, Chien KH, Hung KH, Peng CH, et al. Retinal stem cells and potential cell transplantation treatments. J Chin Med Assoc 77: 556-561.
- Jin ZB, Gao ML, Deng WL, Wu KC, Sugita S, et al. (2019) Stemming retinal regeneration with
pluripotent stem cells. Prog Retin Eye Res 69: 38-56. - Borkowska-Kuczkowska A, Slugocka D, Swiatkowska-Flis B, Boruczkowski D (2019) The use of mesenchymal stem cells for the treatment of progressive retinal diseases: a review. Regen Med 14: 321-329.
- Yang Z, Li K, Yan X, Dong F, Zhao C (2010) Amelioration of diabetic retinopathy by engrafted human adipose-derived mesenchymal stem cells in streptozotocin diabetic rats. Graefes Arch Clin Exp Ophthalmol 248: 1415-1422.
- Rajashekhar G, Ramadan A, Abburi C, Callaghan B, Traktuev DO, et al. (2014) Regenerative therapeutic potential of adipose stromal cells in early stage diabetic retinopathy. PLoS One 9: 84671.
- Mead B, Berry M, Logan A, Scott RA, Leadbeater W, et al. (2015) Stem cell treatment of degenerative eye disease. Stem Cell Res 14: 243-257.
- Holan V, Hermankova B, Kossl J (2017) Perspectives of Stem Cell-Based Therapy for Age-Related Retinal Degenerative Diseases. Cell Transplant 26: 1538-1541.
- Holan V, Hermankova B, Krulova M, Zajicova A (2019) Cytokine interplay among the diseased retina, inflammatory cells and mesenchymal stem cells - a clue to stem cell-based therapy. World J Stem Cells 11: 957-967.
- Wong WL, Su X, Li X, Cheung CM, Klein R, et al. (2014) Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2: 106-116.
- Sergejeva O, Botov R, Liutkeviciene R, Kriauciuniene L (2016) Genetic factors associated with the development of age-related macular degeneration. Medicina (Kaunas) 52: 79-88.
- van Lookeren Campagne M, LeCouter J, Yaspan BL, Ye W (2014) Mechanisms of age-related macular degeneration and therapeutic opportunities. J Pathol 232: 151-164.
- Eandi CM, Alovisi C, De Sanctis U, Grignolo FM (2016) Treatment for neovascular age related macular degeneration: The state of the art. Eur J Pharmacol 787: 78-83.
- Vakalis N, Echiadis G, Pervena A, Deligiannis I, Kavalarakis E, et al. (2015) Intravitreal combination of dexamethasone sodium phosphate and bevacizumab in the treatment of exudative AMD. Sci Rep 5: 8627.
- Barzelay A, Weisthal Algor S, Niztan A, Katz S, Benhamou M, et al. (2018) Adipose-Derived Mesenchymal Stem Cells Migrate and Rescue RPE in the Setting of Oxidative Stress. Stem Cells Int 2018: 9682856.
- Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, et al. (2012) Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 379: 713-720.
- Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, et al. (2015) Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 385: 509-516.
- Kumar A, Midha N, Mohanty S, Chohan A, Seth T, et al. (2017) Evaluating role of bone marrow-derived stem cells in dry age-related macular degeneration using multifocal electroretinogram and fundus autofluorescence imaging. Int J Ophthalmol 10: 1552-1558.
- Limoli PG, Vingolo EM, Morales MU, Nebbioso M, Limoli C (2014) Preliminary study on electrophysiological changes after cellular autograft in age-related macular degeneration. Medicine (Baltimore) 93: 355.
- Limoli PG, Limoli C, Vingolo EM, Scalinci SZ, Nebbioso M. Cell surgery and growth factors in dry age-related macular degeneration: visual prognosis and morphological study. Oncotarget 7: 46913-46923.
- Verbakel SK, van Huet RAC, Boon CJF, den Hollander AI, Collin RWJ, et al. (2018) Non-syndromic retinitis pigmentosa. Prog Retin Eye Res 66: 157-186.
- Fahim A (2018) Retinitis pigmentosa: recent advances and future directions in diagnosis and management. Curr Opin Pediatr 30: 725-733.
- Bruninx R, Lepièce GL (2020) La rétinite pigmentaire [Retinitispigmentosa]. Rev Med Liege 75: 73-74.
- Camara CMFDL, Nanda A, Salvetti AP, Fischer MD, MacLaren RE (2018) Gene therapy for the treatment of X-linkedretinitispigmentosa. Expert Opin Orphan Drugs 6: 167-177.
- Campochiaro PA, Mir TA (2018)The mechanism of cone cell death in Retinitis Pigmentosa Prog Retin Eye Res 62: 24-37.
- Noailles A, Maneu V, Campello L, Lax P, Cuenca N (2018) Systemic inflammation induced by lipopolysaccharide aggravates inherited retinal dystrophy. Cell Death Dis 9: 350.
- Ran X, Cai WJ, Huang XF, Liu Q, Lu F, et al. (2014) 'RetinoGenetics': a comprehensive mutation database for genes related to inherited retinal degeneration. Database (Oxford) 2014: 047.
- Berson EL, Rosner B, Sandberg MA, Hayes KC, Nicholson BW, et al. (1993) A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol 111: 761-772.
- Schwartz SG, Wang X, Chavis P, Kuriyan AE, Abariga SA (2020) Vitamin A and fish oils for preventing the progression of retinitis pigmentosa. Cochrane Database Syst Rev 6: CD008428.
- Ducloyer JB, Meur GL, Cronin T, Adjali O, Weber M (2020) Gene therapy for retinitis pigmentosa. Med Sci (Paris) 36: 607-615.
- Morrow T (2013) Retinal implant brings some sight to profound retinitis pigmentosa patients. Manag Care 22: 54-55.
- Cruz L, Coley BF, Dorn J, Merlini F, Filley E, et al. (2013) The Argus II epiretinal prosthesis system allows letter and word reading and long-term function in patients with profound vision loss. Br J Ophthalmol 97: 632-636.
- Roche PO, Georgiadis A, Smith AJ, Pearson RA, Ali RR (2017) Harnessing the Potential of Human Pluripotent Stem Cells and Gene Editing for the Treatment of Retinal Degeneration. Curr Stem Cell Rep 3: 112-123.
- Tucker BA, Park IH, Qi SD, Klassen HJ, Jiang C, et al. (2015) Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice.PLoS One 6: 18992.
- Pearson RA, Barber AC, Rizzi M, Hippert C, Xue T, et al. (2012) Restoration of vision after transplantation of photoreceptors. Nature 485: 99-103.
- Weiss JN, Levy S (2018) Stem Cell Ophthalmology Treatment Study:bone marrow derived stem cells in the treatment of Retinitis Pigmentosa. Stem Cell Investig 5: 18.
- Wiacek MP, Goslawski W, Grabowicz A, Sobus A, Kawa MP, et al. (2021) Long-Term Effects of Adjuvant Intravitreal Treatment with Autologous Bone Marrow-Derived Lineage-Negative Cells in Retinitis Pigmentosa. Stem Cells Int 2021: 6631921.
- Tuekprakhon A, Sangkitporn S, Trinavarat A, Pawestri AR, Vamvanij V, et al. (2021) Intravitreal autologous mesenchymal stem cell transplantation: a non-randomized phase I clinical trial in patients with retinitis pigmentosa. Stem Cell Res Ther 12:52.
- Alghadyan AA (2011) Diabetic retinopathy - An update. Saudi J Ophthalmol 25: 99-111.
- Wong TY, Cheung CM, Larsen M, Sharma S, Simó R (2016) Diabetic retinopathy. Nat Rev Dis Primers 2: 16012.
- Fiori A, Terlizzi V, Kremer H, Gebauer J, Hammes HP, et al. (2018) Mesenchymal stromal/stem cells as potential therapy in diabetic retinopathy. Immunobiology 223: 729-743.
- Abcouwer SF, Gardner TW (2014) Diabetic retinopathy: loss of neuroretinal adaptation to the diabetic metabolic environment. Ann N Y Acad Sci 1311: 174-190.
- Hernández C, Servat AS, Bogdanov P, Simó R (2017) Diabetic retinopathy: new therapeutic perspectives based on pathogenic mechanisms. J Endocrinol Invest 40: 925-935.
- Rajashekhar G, Ramadan A, Abburi C, Callaghan B, Traktuev DO, et al. (2014) Regenerative therapeutic potential of adipose stromal cells in early stage diabetic retinopathy. PLoS One 9: 84671.
- Mendel TA, Clabough EB, Kao DS, Rice TND, Durham JT, et al. (2013) Pericytes derived from adipose-derived stem cells protect against retinal vasculopathy. PLoS One 8: 65691.
- Ezquer M, Urzua CA, Montecino S, Leal K, Conget P, et al. (2016) Intravitreal administration of multipotentmesenchymal stromal cells triggers a cytoprotective microenvironment in the retina of diabetic mice. Stem Cell Res Ther 7:42.
- Gu X, Yu X, Zhao C, Duan P, Zhao T, et al. (2018) Efficacy and Safety of Autologous Bone Marrow Mesenchymal Stem Cell Transplantation in Patients with Diabetic Retinopathy. Cell Physiol Biochem 49: 40-52.
- Sunness JS (2015) Stem cells in age-related macular degeneration and Stargardt's macular dystrophy. Lancet 386: 29.
- Ramsden CM, Powner MB, Carr AJ, Smart MJ, Cruz L, et al. (2013) Stem cells in retinal regeneration: past, present and future. Development 140: 2576-2585.
- Nasonkin I, Illing M, Koehler MR, Schmid M, Molday RS, et al. (1998) Mapping of the rod photoreceptor ABC transporter (ABCR) to 1p21-p22.1 and identification of novel mutations in Stargardt's disease. Hum Genet 102: 21-26.
- Mata NL, Weng J, Travis GH (2000)Biosynthesis of a major lipofuscinfluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc Natl Acad Sci USA 97: 7154-7159.
- Song WK, Park KM, Kim HJ, Lee JH, Choi J, et al. (2015) Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Reports 4: 860-872.
- Evangelho K, Mogilevskaya M, Barragan ML, Sanchez JKV (2019) Pathophysiology of primary open-angle glaucoma from a neuroinflammatory and neurotoxicity perspective: a review of the literature. Int Ophthalmo l39: 259-271.
- Weinreb RN, Aung T, Medeiros FA (2014)The pathophysiology and treatment of glaucoma: a review. JAMA 311: 1901-1911.
- Khawaja AP, Bailey JNC, Wareham NJ, Scott RA, Simcoe M, et al. (2018) Genome-wide analyses identify 68 new loci associated with intraocular pressure and improve risk prediction for primary open-angle glaucoma. Nat Genet 50: 778-782.
- Hu Y, Tan HB, Wang XM, Rong H, Cui HP, et al. (2013) Bone marrow mesenchymal stem cells protect against retinal ganglion cell loss in aged rats with glaucoma. ClinInterv Aging 8: 1467-1470.
- Limoli PG, Limoli C, Vingolo EM, Franzone F, Nebbioso M (2021)Mesenchymal stem and non-stem cell surgery, rescue, and regeneration in glaucomatous optic neuropathy. Stem Cell Res Ther 12: 275.
Citation: Florido A, Budini M, Vingolo EM (2022) Different Applications of Stem Cells Therapy for Degenerative Retinal Diseases. J Stem Cell Res Dev Ther 8: 093.
Copyright: © 2022 Antonio Florido, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

