
Effects of Position on Intracranial Pressure Management in Porcine Traumatic Brain Injury with Hemorrhagic Shock
*Corresponding Author(s):
Kyoung Jun SongDepartment Of Emergency Medicine, Seoul National University Boramae Medical Center, 20 Boramae-Ro 5 Gil, Dongjak-gu, Seoul, 07061, Republic Of Korea
Tel:+82-2-870-2662,
Fax:+82-2-831-0207
Email:skciva@gmail.com
Abstract
Objectives: Traumatic Brain Injury (TBI) and hemorrhagic shock have high mortality rates. These two conditions are frequently associated with multiply-compromised patients. The patient's position affects blood pressure and Intracranial Pressure (ICP). The goal of this study was to determine the effect of posture on the treatment of patients with TBI and shock.
Methods: This study utilized a randomized experimental design and anesthetized, intubated and paralyzed female pigs (n=12) (mean 46.3±2.4 kg). Whole pigs were divided into supine, reverse Trendelenburg and Trendelenburg groups, and four pigs were assigned to each group. After reaching a target Systolic Blood Pressure (SBP) of less than 60 mmHg, and Intracranial Pressure (ICP) of more than 50mmH, the position was changed according to the designated group and observed for one hour. The main outcome was the Cerebral Perfusion Pressure (CePP), and the secondary outcome was the Mean Aortic Blood Pressure (MBP). The outcomes were compared using a mixed model analysis.
Results: One pig in the reverse Trendelenburg group died during induction and a total of 11 pigs were analyzed. All three pigs assigned to the head elevation group died during the observation period, and none of the other pigs died. There was no significant difference in CePP among three groups (p=0.932). MBP was significantly lowest in the reverse Trendelenburg group and significantly highest in the Trendelenburg group compared with the other groups (p<0.0167). ICP and Coronary Perfusion Pressure (CoPP) were lower in the reverse Trendelenburg group than the supine group (p<0.0167).
Conclusion: The head elevation position increased mortality, decreased MAP, and showed no significant change in CePP.
Keywords
Traumatic brain injury; Intracranial pressure; Position
Introduction
Traumatic injury is a major public health problem. As a result of injury, about 4.7 million people die each year, and this accounts for 8.5% of all deaths [1]. Traumatic brain injury with hemorrhagic shock is one of the major causes of death due to severe trauma. The most common cause of acute phase death in trauma-related deaths is hemorrhagic shock, and the most common cause of delayed death is traumatic brain injury [2,3]. In addition, 6-16% of deaths due to trauma are accompanied by hemorrhagic shock and traumatic brain injury [4]. Therefore, it is important to reduce mortality in patients who have traumatic brain injury with hemorrhagic shock.
In patients with hemorrhagic shock and in patients with traumatic brain injury, position is one of the important therapeutic interventions that can be performed immediately and is a significant factor in the hemodynamic index. However, the recommended positions for patients with hemorrhagic shock and traumatic brain injury are different, and it is not well known which position should be applied to patients with both.
Previous studies have been conducted on how the position of the patient affects traumatic brain injury and hemorrhagic shock. The head elevation position is commonly used to reduce increased Intracranial Pressure (ICP) in TBI patients [5]. In patients with traumatic brain injury, it is important to prevent IICP and maintain adequate CBF to prevent secondary brain injury [6-8]. In theory, head elevation is effective because it improves cerebral venous return and reduces ICP by promoting the distribution of CSF into the subarachnoid spinal space area [9]. However, there is no evidence that head elevation is effective in recent studies [10]. In patients with hemorrhagic shock, the Trendelenburg position has been used to improve transient hypotension [11]. However, there is no evidence that the Trendelenburg position is effective from previous studies [12]. It is also unclear which position is most appropriate for patients with traumatic brain injury with hemorrhagic shock. In patients with hemorrhagic shock, head elevation may reduce venous return, resulting in decreased cardiac output and decreased systemic pressure.
We hypothesized that the head elevation position would reduce blood pressure and therefore reduce the CePP. We investigated which position is most appropriate in the porcine increased ICP with hemorrhagic shock model. Through this experiment, we will be able to develop clinical guidelines for patients with multiple trauma with TBI and shock.
Materials And Methods
Study design and setting
A randomized controlled animal experimental study was designed. Twelve domestic pigs weighting 40-50 kg and aged three months were used in the experiment. The IICP with hemorrhagic shock model was made using blood loss of the femoral vein and intracranial space occupying lesion. The experimental animals were randomly divided into a supine group, head elevation group, and head down group. Each group was observed for one hour after changing posture.
The sample size was calculated by assuming that the difference in CePP of each group was 10mmHg and the standard deviation was 5 mmHg, based on a previous study on the difference of CePP according to posture in cardiac arrest. The calculation demonstrated that to achieve the study power of 95% and alpha risk level of 0.05, a sample size of four animals was required in each group (i.e., a total of twelve animals).
Surgical preparation
Pigs were positioned on a tilt with their legs tied to the ends of the tilt table. Pigs were initially sedated with intramuscular injection of 5 mg/kg of tiletamin/zolazepamhypochloride (Zoletil®, Virbac laboratories, France) and 2 mg/kg ofxylazine (Rompun®, BayerKorea, South Korea), followed by inhaled isoflurane at a dose of 1-1.5%. While sedated and ventilated, tracheal intubation was performed using a 7.5 mm internal diameter endotracheal tube. Pigs were ventilated with a volume-control ventilator adjusted at a tidal volume of 6-8ml/kg, a respiratory rate adjusted to continually maintain end-tidal CO2 (ETCO2) of 40 mmHg and O2 saturation above 94%. After creating a burr hole at the middle distance between the occipital bony prominence and the right eyebrow, a micromanometer-tipped catheter (Mikro-Tip®transducer, Millar Inc., Houston, TX, USA) was inserted into the parietal lobe to measure Intracranial Pressure (ICP). Another burr-hole with a 5 mm diameter was made at the middle of the distance between the occipital bony prominence and the left eyebrow. Then, an 18 Fr Foley catheter with 30 cc ballooning was inserted through the burr hole into the right parietal lobe. A second Mikro-tip®Trans-ducer was inserted through the left femoral artery under ultrasonic guidance and placed in the descending thoracic aorta to measure the aortic blood pressure. A 3-lumen central catheter was inserted into the right femoral vein for blood loss. Another Mikro-tip®Transducer was inserted to the right atrium via the right external jugular vein to record the right atrial pressure. The position of the transducer was confirmed by characteristic pressure tracings. Carotid Blood Flow (CBF) was measured with a one channel perivascular flowmeter (T420, Transonic Systems Inc., Ithaca, NY, USA) with the implantable perivascular flow probe (MA-2PSS, Transonic Systems Inc., Ithaca, NY, USA) placed on the internal carotid artery. Open cystostomy was performed and an 18 Fr foley catheter was placed in the bladder. The animals fasted overnight. The pigs’ rectal temperature was maintained between 37 °C to 38 °C using a warming blanket (Blanketrol®II, Cincinnati Sub-Zero Medical Division, Cincinnati, OH, USA) throughout the duration of the experiment. Additionally, Electrocardiography (ECG), Oxygen Saturation (SpO2) monitoring from the earlobe surface, and end tidal CO2 (ETCO2) levels were monitored and recorded throughout the experiment. All data measurements were recorded using digital data acquisition software (PowerLab®, ADInstruments Inc., Colorado Springs, CO, USA).
Experimental protocol
The experimental animals were randomly divided into a supine position group, Reverse Trendelenburg Position group (RTP) and Trendelenburg Position group (TP). After surgical preparation, the increased ICP model with hemorrhagic shock was induced. In this study, when systolic blood pressure reached 60 mmHg and ICP reached 50mmHg and was maintained for one minute, posture changes were made. A femoral venous catheter was used to induce blood loss, and a 30 cc 16 Fr foley catheter was inserted into the burr hole to induce space-occupied lesion. Considering the elevation of blood pressure due to Cushing's reflex, blood loss and elevated intracranial pressure were simultaneously achieved. Blood loss was conducted at a rate of 50cc/2 min, and Foley catheter inflation proceeded at a rate of 1.5cc/2 min. After systolic blood pressure and ICP reached the target value, position changes were achieved with a specially customized tilt table which is locked at a designated tilt degree. For the head elevation group, we fixed the tilt table at +30°, and for the head down group we fixed the tilt table at -30°. After the position change, 20% mannitol 0.5g/kg was infused via a central catheter. The hemodynamic parameters were measured continuously for one hour. Complete Blood Count (CBC), Arterial Blood Gas Analysis (ABGA) and serum lactate level were measured via femoral catheter before and after the change of position, at 20, 40 and 60 minutes. Upon completion of the experiment protocol, pigs were then sacrificed with a 20 ml injection of saturated potassium chloride (Figure 1).
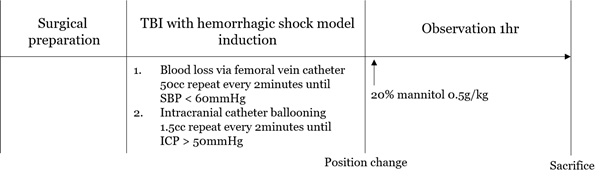 Figure 1: Experimental protocol.
Figure 1: Experimental protocol.
Outcome measures
The primary outcome is the CePP difference according to the change of posture during the observation period, and the secondary outcome is MAP, CoPP, and CBF, which are indicators of mortality and systemic circulation of the brain circulation system during the posture observation.
Variables
Aortic blood pressure, right atrial blood pressure, and intracranial pressure were collected through a micromanometer-tipped catheter during the study period. CePP was calculated using the difference between aortic blood pressure and intracranial pressure. CoPP was calculated as the difference between the aortic blood pressure and the right article blood pressure. CBF was obtained by integrating carotid blood flow for one minute. Each value was obtained in real time, and the value was extracted for ten seconds every one minute for analysis. To compare blood loss, hemoglobin levels were measured at baseline, immediately after position change, and after the end of the experiment. Serum lactate level was measured at baseline, immediately after position change, and after 20 minutes, 40 minutes, and 60 minutes.
Statistical analysis
Data are expressed as mean and Standard Deviation (SD). Continuous variables including weight, blood loss, intracranial ballooning, induction time, Hb, Hct, and lactate levels were compared by Kruskal-Wallis test. The p-value of each group was significant when it was lower than 0.0167 by Bonferroni-correction. The difference of the hemodynamic parameters with time according to position was compared with the mixed model analysis. Bonferroni-correction was used for post hoc analysis, and a p-value of less than 0.0167 was considered significant. All statistical analysis was performed using SAS 9.4 software.
Ethics statements
This study was approved by the Institutional Animal Care and Use Committee of the study institution. All animal care was compliant with the Laboratory Animal Act of the Korean Ministry of Food and Drug Safety. A certified and licensed veterinarian assured that the protocols were performed in accordance with the aforementioned guidelines.
Results
A total of 12 pigs were tested. One pig in the supine group expired during the induction process so the result was analyzed through 11 pigs. Table 1 shows the baseline characteristics of the experimental animals included in the analysis. There was no statistically significant difference in mean blood loss among each group (RTP, mean(SD): 590(179)cc, SP: 683(76)cc, TP: 463(132)cc, p= 0.20) and ballooning volume (RTP: 44.8(1.3)cc, SP: 46.3(2.5)cc, TP: 48(2.6)cc, p = 0.21) for each position. And, there was no significant difference in model induction time (RTP: 22.8(9.5)min, SP: 28.0(2.9)min, T: 26.3(19.9)min, p-value = 0.52). In the experiment, all pigs assigned to the reverse Trendelenburg group expired before 25 minutes and the other pigs assigned to other groups did not expire.
The change in hemodynamic parameters with time is shown in Table 2. Baseline hemodynamic parameters were not significantly different between groups. SBP was significantly lower in RTP than other groups (p <0.0167), and TP was significantly higher than other groups (p <0.0167). DBP and MAP were significantly lower in RTP than SP and TP (p <0.0167). RAP did not show significant differences among three groups. The ICP was significantly lower in RTP than in SP (p <0.0167). CePP did not show significant difference among the three groups. CoPP had a significantly lower RTP than SP (p <0.0167). In CBF, RTP was significantly lower than SP and TP. (p <0.0167).
|
Variables |
All |
Group |
p-value |
||
|
RTP |
SP |
TP |
|||
|
Weight, |
46.7(2.5) |
46.8(2.8) |
45.4(2.1) |
48.0(2.6) |
0.35 |
|
mean(SD) (Kg) |
|||||
|
Hemorrhage, mean(SD) (cc) |
569(157) |
683(76) |
590(179) |
463(132) |
0.2 |
|
Intra-cranial ballooning, |
12.3(7.1) |
12.8(6.5) |
16.2(8.8) |
8.1(4.1) |
0.16 |
|
mean(SD) (cc) |
|||||
|
Induction time, mean(SD) (min) |
26.0(11.7) |
27.7(3.5) |
24.5(7.0) |
26.3(19.9) |
0.43 |
|
Mortality |
04-Nov |
0/3 |
04-Apr |
0/4 |
<0.05 |
Table 1: Baseline model induction variables and mortality.
RTP: Reverse Trendelenburg Position; SP: Supine Position; TP: Trendelenburg Position; SD: Standard Deviation.
|
Parameter |
Time(min) |
p-value* |
||||||||
|
Baseline |
1 |
5 |
10 |
15 |
20 |
40 |
60 |
|||
|
SBP, mmHg |
|
<0.001 a,b,c |
||||||||
|
|
RTP |
55(3) |
27(22) |
39(7) |
35(6) |
23(21) |
|
|||
|
|
SP |
55(4) |
51(2) |
60(7) |
57(8) |
52(3) |
48(2) |
44(3) |
41(4) |
|
|
|
TP |
53(3) |
60(7) |
66(9) |
63(12) |
60(10) |
58(9) |
55(7) |
56(8) |
|
|
DBP, mmHg |
<0.001 a,c |
|||||||||
|
RTP |
33(5) |
15(17) |
20(6) |
19(7) |
11(14) |
|||||
|
SP |
31(8) |
29(7) |
31(11) |
27(6) |
27(6) |
27(6) |
24(4) |
25(5) |
|
|
|
TP |
31(5) |
35(5) |
38(6) |
37(9) |
36(8) |
35(6) |
33(4) |
33(5) |
||
|
MAP, mmHg |
<0.001 a,c |
|||||||||
|
RTP |
40(4) |
19(18) |
26(6) |
24(7) |
15(16) |
|||||
|
SP |
40(6) |
37(5) |
41(9) |
37(4) |
36(4) |
35(5) |
31(3) |
30(4) |
|
|
|
TP |
39(5) |
43(6) |
47(7) |
46(9) |
45(8) |
43(7) |
40(5) |
41(6) |
||
|
RAP, mmHg |
0.317 |
|||||||||
|
RTP |
2(4) |
-1(7) |
-2(7) |
-2(6) |
-2(6) |
|||||
|
SP |
3(3) |
3(3) |
3(3) |
3(3) |
4(3) |
4(2) |
2(2) |
2(4) |
|
|
|
TP |
6(8) |
7(2) |
6(3) |
6(3) |
7(3) |
7(3) |
7(3) |
6(2) |
|
|
|
ICP, mmHg |
0.020 a |
|||||||||
|
RTP |
60(9) |
43(7) |
26(6) |
23(7) |
18(6) |
|
||||
|
SP |
52(2) |
49(2) |
45(3) |
43(9) |
40(7) |
37(6) |
35(10) |
27(3) |
|
|
|
TP |
61(10) |
60(4) |
50(8) |
46(8) |
43(9) |
41(10) |
38(11) |
38(12) |
|
|
|
CePP, mmHg |
0.932 |
|||||||||
|
RTP |
-19(13) |
-23(17) |
-1(12) |
0(12) |
-4(15) |
|
||||
|
SP |
-14(7) |
-13(8) |
-6(11) |
-8(12) |
-5(8) |
-3(7) |
-4(8) |
3(4) |
|
|
|
TP |
-21(15) |
-16(9) |
-2(10) |
1(12) |
2(12) |
2(10) |
2(10) |
3(9) |
|
|
|
CoPP, mmHg |
0.020 a |
|||||||||
|
RTP |
42(7) |
21(12) |
31(6) |
28(4) |
18(14) |
|
||||
|
SP |
40(13) |
35(13) |
38(17) |
35(13) |
34(13) |
33(13) |
33(13) |
32(16) |
|
|
|
TP |
34(7) |
39(9) |
43(10) |
42(12) |
40(12) |
39(12) |
36(13) |
37(14) |
|
|
|
CBF, L/min |
<0.001 a,c |
|||||||||
|
RTP |
5.1(1.2) |
2.5(1.9) |
0.5(0.3) |
0.3(0.2) |
0.1(0.2) |
|
||||
|
SP |
4.5(0.7) |
4.5(0.6) |
5.6(1.4) |
5.6(0.8) |
5.1(0.7) |
4.9(0.6) |
4.4(0.5) |
4.1(1.6) |
|
|
|
TP |
4.9(1.7) |
6.4(1.1) |
7.3(1.1) |
6.9(0.9) |
6.3(0.5) |
6.1(0.2) |
5.9(0.4) |
5.9(0.9) |
|
|
Table 2: Hemodynamic parameters changing according to each position
RTP: Reverse Trendelenburg Position; SP: Supine Position; TP: Trendelenburg Position; SBP: Systolic Aortic Blood Pressure; DBP: Diastolic Aortic Blood Pressure; MAP: Mean Aortic Blood Pressure; RAP: Right Artrial Blood Pressure; ICP: Intracranial Pressure; CePP: Cerebral Perfusion Pressure; CoPP: Ccoronary Perfusion Pressure; CBF: Carotid Blood Pressure.; a , P-values significant after Bonferroni correction (<0.0167) between supine group and reverse trendelenburg group; b , P-values significant after Bonferroni correction (<0.0167) between supine group and trendelenburg group; c , P-values significant after Bonferroni correction (<0.0167) between reverse trendelenburg group and trendelenburg group.
Immediately after position change, MAP remained constant throughout the observation period in the TP and SP groups, but did not recover after reduction immediately after conversion in the RTP group (Figure 2a). ICP was found to decrease with time in all three groups, but especially in the RTP group (Figure 2b). CePP increased similarly in the entire group regardless of positional change (Figure 3a). CoPP remained constant throughout the observation period in the SP and TP groups and decreased gradually over time in the RTP group (Figure 3b).
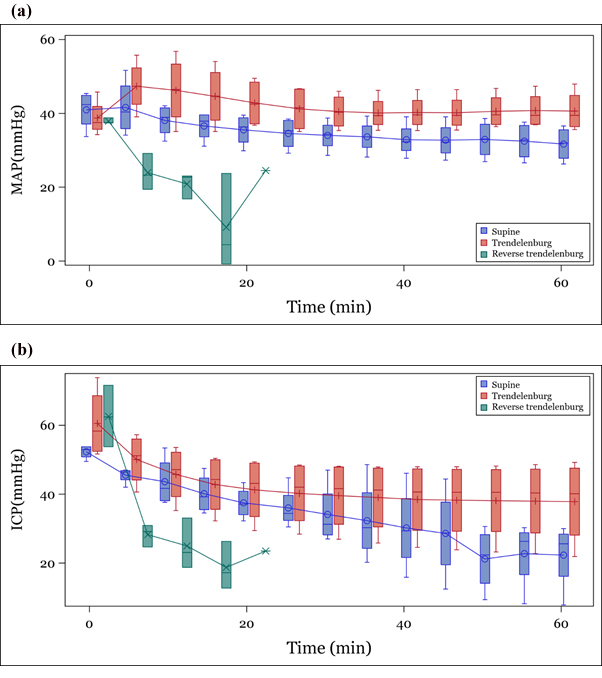 MAP: Mean Aortic Arterial Pressure; ICP: Intracranial Pressure
MAP: Mean Aortic Arterial Pressure; ICP: Intracranial Pressure
Figure 2: Trend of parameters according to position (a) MAP, (b) ICP.
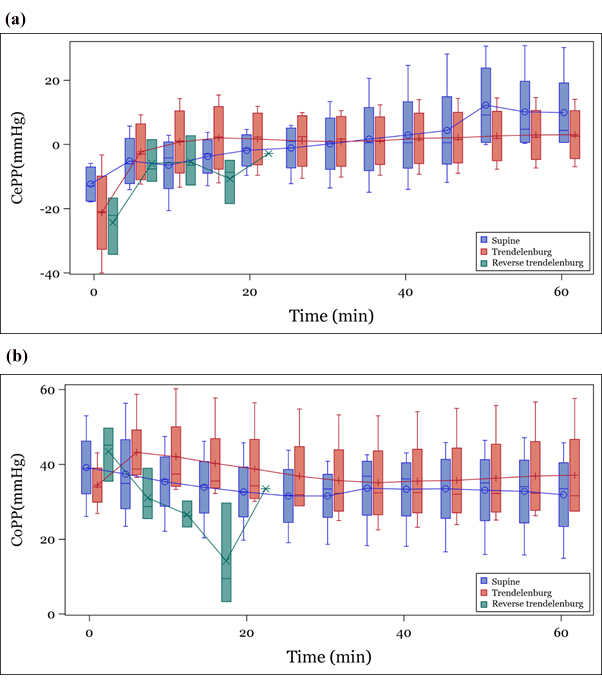 CePP, cerebral perfusion pressure; CoPP, coronary perfusion pressure
CePP, cerebral perfusion pressure; CoPP, coronary perfusion pressure
Figure 3: Trend of calculated parameters according to position (a) CePP, (b) CoPP.
The laboratory test changes are shown in Table 3. Hb and hematocrit, which are indicative of blood loss, were not significantly different among the three groups. The decrease in hemoglobin before and after the induction of blood loss also showed no significant difference (p=0.37). The amount of lactate level difference from basal to end in the RTP group (4.0 mmol/L) was significantly higher than that of the Trendelenburg (0.5 mmol/L) and supine groups (0.5 mmol/L) (p=0.03).
|
Laboratory test |
Position |
p-value |
|||
|
Reverse trendelenburg |
Supine |
trendelenburg |
|||
|
Hb (g/dL), Mean(STD) |
|
|
|
|
|
|
Basal |
9.9(1.9) |
10.1(1.1) |
10.6(0.8) |
0.75 |
|
|
After Induction |
11.1(0.9) |
11.2(1.9) |
10.3(0.8) |
0.41 |
|
|
End |
11.0(3.0) |
9.3(0.7) |
9.4(1.2) |
0.39 |
|
|
|
Δ(End-Basal) |
1.1(3.7) |
-0.8(0.5) |
-1.2(2.0) |
0.37 |
|
Hct (%), Mean(STD) |
|
|
|
||
|
Basal |
33.0(5.5) |
27.8(1.7) |
30.8(4.3) |
0.22 |
|
|
After Induction |
32.9(3.6) |
30.3(1.6) |
29.0(4.6) |
0.39 |
|
|
20min |
35.0(1.8) |
27.7(4.5) |
26.8(2.5) |
0.03 |
|
|
40min |
|
26.7(2.5) |
26.3(1.0) |
0.28 |
|
|
60min |
|
26.7(3.5) |
27.8(1.9) |
0.72 |
|
|
Δ(End-Basal) |
3.8(7.7) |
-1.1(4.5) |
-3.0(3.6) |
0.23 |
|
|
Lactate(mmol/L), |
|
|
|
||
|
Basal |
1.7(0.3) |
2.5(1.2) |
2.0(0.7) |
0.55 |
|
|
After Induction |
2.6(1.0) |
2.8(2.2) |
2.4(0.8) |
0.91 |
|
|
20min |
5.2(1.3) |
3.0(2.1) |
2.3(0.9) |
0.09 |
|
|
40min |
|
2.8(1.7) |
2.2(0.9) |
0.31 |
|
|
60min |
|
3.0(1.6) |
2.5(1.1) |
1 |
|
|
Δ(End-Basal) |
4.0(1.8) |
0.5(1.2) |
0.5(0.9) |
0.03 |
|
Table 3: Laboratory test result according to each position groups.
Discussion
Traumatic injury is a major health problem that accounts for about 8.5% of all deaths. 1 Among them, traumatic brain injury with hemorrhagic shock accounts for 6-16% of all trauma deaths [4]. The patient's position is one of the important therapeutic factors causing hemodynamic change in the patients who have traumatic brain injury with hemorrhagic shock. This study was conducted to investigate the hemodynamic change of the patient's posture using the traumatic brain injury with hemorrhagic shock model. This study showed that head elevation position causes intracranial pressure reduction with traumatic brain injury with severe hemorrhagic shock. Accompanied by a decrease in mean arterial pressure affecting cerebral perfusion, it was observed that it did not improve cerebral perfusion. In addition, it was observed that the carotid artery blood flow decreased and the shock of the whole body worsened, increasing the mortality rate.
In patients with traumatic brain injury, the head elevation position is an attempt to reduce brain pressure and is mainly applied to patients with severe traumatic brain injury. In theory, the head elevation posture affects venous return and redistribution through the subarachnoid space of cerebrospinal fluid [9,12]. However, according to the recent meta-analysis of J Alarcon, et al., the combination of three RCTs in patients with severe traumatic brain injury has a significant effect on patient mortality. The level of evidence is low and its effects on ICP and cerebral perfusion are not clear [10]. But, this study shows that in patients with TBI with hemorrhagic shock, the head elevation position immediately reduced ICP. However, as a result, it was shown that the MAP reduced and resulted in no significant change in CePP.
Previous studies have shown that in patients with low blood pressure shock, the Trendelenburg position shifts the lower extremity blood flow centrally due to autotransfusion effects, resulting in a temporary increase in cardiac output of about 10% [13]. However, the effect of the Trendelenburg position is not sustained, and treatment of hypovolemic shock should be prior to fluid therapy. Repositioning is limited in the absence of fluid therapy [12]. The results of this study also showed that the Trendelenburg posture caused a temporary increase in mean arterial pressure in patients with low blood shock with traumatic brain injury, but did not show a statistically significant difference. The Trendelenburg position did not significantly increase intracranial pressure or decrease cerebral perfusion pressure with traumatic brain injury. However, the cerebral perfusion pressure tended to be higher than that of the supine position between ten and forty minutes. This may be due to the effect of a transient blood pressure increase in the Trendelenburg position in patients with traumatic brain injury with severe hemorrhagic shock.
In this study, all three animals in the reverse Trendelenburg group died within 20 minutes. The animal model used in this experiment maintained ICP at 50 mmHg and systolic arterial pressure at 60 mmHg or less. Increased ICP is known to be accompanied by increased blood pressure, irregular breathing, and bradycardia - called the Cushing reflex. In spite of this Cushing reflex, the decreased blood pressure is likely due to catecholamine release due to the Cushing reflex, which has already been accompanied by vasoconstriction and a reduced ability to cope with relative shock deterioration due to head elevation. And reverse Trendelenburg position may lead to aggravation of hemorrhagic shock, and death. This suggests that the actual severity of hemorrhagic shock may be underestimated, even if blood pressure is stable in patients with traumatic brain injury.
Limitations
There are several limitations in this study. First, it was based on space-occupying lesions using intra-cranial ballooning to induce increased intracranial pressure. However, the intra-cranial ballooning model differs from actual traumatic brain injury. It does not effectively induce the effects of brain-blood barrier damage, brain parenchymal damage such as cerebral contusion, and accompanying brain edema. Second, this study used a pig model, but there are several anatomical differences between pigs and humans. The brains of pigs are only 96-145 g, which is only one tenth the size of human brains, which weigh about 1.2 kg. Thus, intracranial balloon models are more likely to cause cerebral herniation. Third, isoflurane was used to maintain the sedation of the pig in this experiment. Previous studies have reported isoflurane-induced hypotension by reducing vascular resistance, which may lead to worsening of hypotension [14]. Finally, this study did not administer any intra-venous fluids during the one-hour observation period to rule out the effects of fluid resuscitation. However, the most important treatment for hemorrhagic shock is the recovery of systemic perfusion with the administration of fluid and blood products. also, immediate fluid therapy is performed at the prehospital phase. Therefore, experiments lacking fluid administration may not show results in the clinical environment in which the fluid is administered along with the actual position change.
Conclusion
In this experiment, the head elevation position increased mortality, decreased MAP, and showed no significant change in CePP. This study suggests that resuscitation from hypotension status is important in maintaining CePP and decreasing the mortality of patients with traumatic brain injury and hemorrhagic shock.
References
- GBD 2015 Mortality and Causes of Death Collaborators (2016) Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388: 1459-1544.
- Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, et al. (1995) Epidemiology of trauma deaths: a reassessment. J Trauma 38: 185-193.
- Sobrino J, Shafi S (2013) Timing and causes of death after injuries. Proc (Bayl Univ Med Cent) 26: 120-123.
- Kauvar DS, Lefering R, Wade CE (2006) Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma 60: S3-S11.
- Sullivan J (2000) Positioning of patients with severe traumatic brain injury: research-based practice. J Neurosci Nurs 32: 204-209.
- Ghajar J (2000) Traumatic brain injury. Lancet 9233: 923-929.
- Ortega-Pérez S, Amaya-Rey MC (2015) Secondary brain injury: a concept analysis. J Neurosci Nurs 50: 220-224.
- Carney N, Totten AM, O’Reily C, Ullman JS, Hawryluk GW, et al. (2017) Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 80: 6-15.
- Fan JY (2004) Effect of backrest position on intracranial pressure and cerebral perfusion pressure in individuals with brain injury: a systematic review. J Neurosci Nurs 36: 278-88.
- Alarcon JD, Rubiano AM, Okonkwo DO, Alarcon J, Martinez-Zapata MJ, et al. (2017) Elevation of the head during intensive care management in people with severe traumatic brain injury. Cochrane Database Syst Rev 12: CD009986.
- Martin JT (1995) The Trendelenburg position: a review of current slants about head down tilt. AANA J 63: 29-36.
- Bridges N, Jarquin-Valdivia AA (2005) Use of the Trendelenburg position as the resuscitation position: to T or not to T? Am J Crit Care 14: 364-368.
- Geerts BF, van den Bergh L, Stijnen T, Aarts LP, Jansen JR (2012) Comprehensive review: is it better to use the Trendelenburg position or passive leg raising for the initial treatment of hypovolemia? J Clin Anesth 24: 668-674.
- Newberg LA, Milde JH, Michenfelder JD (1984) Systemic and cerebral effects of isoflurane-induced hypotension in dogs. Anesthesiology 60: 541-546.
Citation: Choi DS, Song KJ, Kim TH, Chang IW, Ro YS, et al. (2021) Effects of Position on Intracranial Pressure Management in Porcine Traumatic Brain Injury with Hemorrhagic Shock. J Emerg Med Trauma Surg Care 8: 059.
Copyright: © 2021 Dong Sun Choi, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

