
Evaluation of Antidiabetic and Histopathological Activities of Embelia robusta Seeds Extract against Alloxan-Induced Diabetic Wistar Rats
*Corresponding Author(s):
Sharada SeekondaaDepartment Of Genetics, Osmania University, Hyderabad, India
Tel:+040 23017058,
Email:sharada.msc@gmail.com
Abstract
Objective
To investigate the anti-diabetic activity of methanolic seed extract of Embelia robusta (E.robusta) on Alloxan (130 mg/kg body weight) induced diabetic rats.
Method
The oral administration of 30 adult wistar rats was randomly divided into 5-groups of 6 rats each and received the following treatments: Group-I: Normal healthy control rats treated with vehicle alone (distilled water), Group-II: Diabetic control rats treated with vehicle alone (distilled water), Group-III: Diabetic rats treated with standard Gliclazide drug with 50 mg/kg, Group-IV: Diabetic rats treated with 50 mg/kg of methanolic seed extract of E.robusta, Group-V: Diabetic rats treated with 100 mg/kg of methanolic seed extract of E.robusta. The freshly prepared solutions of the extract were orally administered using an intragastric tube once daily for 28 days, continuously.
Results
The results were significantly lowered in the test groups of IV &V with 50 and 100 mg/kg of seed extract of E.robusta. The percentages of blood glucose levels decreased by 41.94% and 53.84%, those were comparable to the test group of III with standard gliclazide drug (61.97%). In the oral glucose tolerance test, seeds extract of E.robusta treatment significantly increased (p < 0.05) the glucose tolerance compared with the vehicle control. The improved glucose metabolism of seed extract of E.robusta treated rats could be due to better insulin secretion from β-cells and an augmented transport and utilization of glucose than those of diabetic rats.
Conclusion
These results suggest that the methanolic seed extract of E.robusta possess has anti-diabetic activity, which is comparable to the standard drug, as such, a potentially important compound in anti-diabetic drug discovery.
Keywords
Diabetes; Embelia robusta; Fasting Blood Glucose (FBS); Gliclazide; Histopathology
Introduction
Diabetes mellitus is a metabolic disorder, characterized by hyperglycemia with disturbances of carbohydrate, fat and protein metabolism resulting from defects in insulin secretion, insulin action, or both. The burden of diabetes is increasing globally and estimates suggest that there will be 366 million cases by the year 2030, with increases particularly in developing countries [1,2]. Diabetes is a complex metabolic disorder associated with pathogenesis of macrovascular (cardiomyopathy, diabetic foot diseases) and microvascular (diabetic retinopathy, neuropathy and nephropathy) complications. The increased blood glucose levels can increase oxidative stress by several mechanisms, including glucose auto-oxidation, non-enzymatic protein glycation and activation of polyol pathway, resulting in the development of chronic complications in diabetes [3,4]. Diabetes is also a clinical syndrome characterized by hyperglycaemia due to absolute or relative deficiency of insulin. This can arise in many different ways, but is most commonly due to autoimmune type-1 diabetes or to adult onset type-2 diabetes. It develops when the body tissue or cells can no longer recognize insulin for sugar metabolism. As such, glucose is not metabolised and high amount of it remains in circulation (hyperglycaemia). Two major roles of insulin are to promote the breakdown of glucose and synthesis of glycogen as storage of the excess sugar [5]. However, insulin resistance causes a decrease in the synthesis of glycogen and alters the activity of enzymes involving in glycogen synthesis [6]. Lack of insulin affects the metabolism of carbohydrates, proteins and fats, and can cause a significant disturbance of water and electrolyte homeostasis [7]. The long standing diabetic condition affects the other organs like eye, skin, kidney, nervous system and macro, micro vascular complications [8].
Diabetes are mostly being managed by keeping the blood sugar level as close to normal with drugs such as metformin, gliclazide, sulfonylurea and thiazolidinediones etc. as well as exogenous insulin [9,10]. However, some of these oral medications have been shown to have side effects. Gliclazide causes gastrointestinal side effects and lactic acidosis, sulfonylurea causes hypoglycaemia and weight gain, while thiazolidinediones causes cognitive heart failure, fluid retention and bone fractures and exogenous insulin has also been shown to cause weight gain and hypoglycaemia [11]. More so, based on the influence of oxidative stress on diabetes complications, more effective methods of management will entail antidiabetic as well as antioxidants treatments [12]. Insulin has proved to be effective to some extent in increasing the life expectancy of diabetic patients, but is not a permanent solution since there are many drawbacks of this therapy. Also the therapy with oral hypoglycaemic agents is not satisfactory. Traditional medicine is gaining so much interest recently due to their multiple modes of actions with minimal adverse effects in humans [13]. Thus, the search for a new therapeutical agent devoid of adverse effect originating from plants used in traditional medicine would be of interest.
Medicinal plants have richest sources of bioactive compounds which can be extracted and screened in the diabetic research. There are numerous traditional medicinal plants reported to have hypoglycaemic properties, such as Gymnema sylvestre [14], Embelica officianalis [15], Momordica charantia, Carica papaya,Trigonella foenum, Curcuma longa [16], Embelia robusta [17], Caesalpinia bonducella [18], and Pithecellobium dulce [19]. Among them Embelia robustais a well-known drug in Ayurvedic system of medicine. Embelia robustais described since Vedic period as an important large climbing herb. It is an important species under the family of Myrsinaceae, commonly known as vidanga and vayavidang. For the first time, Embelia ribes and Embelia robusta were botanically described as Baiburang by Roxburgh. The leaves, berries, seeds and root barks are-employed in various formulations singly or in composite formulations. It occurs in the forests of Kerala and is collected for manufacturing of medicines, dyes cosmetics and other products [20]. The dried fruit mainly constitutes the drug, which is used as an anthelmintic, carminative, stimulant and alternative. Seeds of E. robusta have potent medicinal value as they are used for the preparation of various traditional drugs which are prescribed in headache, rhinitis, hemicranias, epilepsy, insomania, and anthelmintic, etc. The E.robusta is a climber with slender branches and long internodes. The leaves are elliptic broad and covered with minute glands. The fruits are berries and contain the benzoquinone compound, embelin [2,5-dihydroxy-3-undecyl,2-cyclohexadiene-1,4-benzo-quinone] as a major bioactive constituent. The seed extract of E.robusta shows various biological activities such as anticancer, antifungal, antibacterial [21], and free radical scavenging [22].The power of seeds is also directly applied in ringworm. It is also used for the prevention of pregnancy and hair dying.
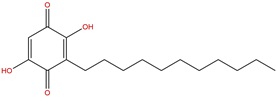 Structure of Embelin
Structure of Embelin
In Ayurveda, E.robusta has been acknowledged to treat various diseases and disorders, including diabetes. However, little or no study has evaluated the combined antioxidant and anti diabetic activities of the seed extracts of E.robusta. Therefore, the present work was an attempt to evaluate the antidiabetic activity of the seeds of plant (seed extracts of E.robusta) and to generate scientifically justified data to support the activity. In particular, the present study was carried out to investigate the effects of E.robusta seed extract on blood glucose level and histopathological changes in alloxan induced diabetic rats.
Materials and Methods
Chemicals
Methanol was purchased from Sigma Aldrich chemicals, India. Gliclazide was collected from Pharmacy division, Hyderabad. E.robusta seeds were collected from local market identified and authenticated by Department of Botany, Osmania University College for Women, Koti, Hyderabad. All other chemicals and reagents used were of analytical grade.
Preparation of E.robusta seed extract
The fresh seeds of E.robusta were collected during the month of July 2019, from Hyderabad, India. Freshly collected seeds were cleaned by removing adhering dust and arils, and then dried under shade. The 100 g of dried samples were powdered and successively extracted with petroleum ether (60-80°C) and methanol (70%) using Soxhlet apparatus for 72 h. The methanol extract was concentrated by rotary vacuum evaporator and then dried. The obtained methanolic extract of E.robusta was stored in the refrigerator at 4°C until further use. For further animal study, the different concentrations of seed extracts 50 and 100 mg/kg was made by dissolving in 1% Tween 80 in the normal saline.
Experimental animals
The healthy adult albino Wistar rats (150-200 g) were procured from the National Institute of Nutrition (NIN), Hyderabad, India. The animals were housed in standard polypropylene cages and maintained under controlled room temperature (22 ± 2°C) and humidity (55 ± 10%) with 12 h light/dark cycle. All the rats were provided with standard pellet diet, ad libitum and had free access to water. The guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) Govt. Of India were followed and prior permission was sought from the Institutional Animal Ethics Committee (IAEC), for conducting this study (Registration no. 428/01C/CPCSEA, 7 February 2015, Gandhi Medical College, Hyderabad).
Experimental design and induction of diabetes in rats
Experimental diabetes was induced in all groups except vehicle control following overnight fasting (deprived of food for 12 h allowed free access to water) by a single in traperitonial injection of 50 mg/kg of Alloxan dissolved in a freshly prepared 0.1 M cold citrate buffer (pH 4.5). Alloxan-injected animals were given 5% glucose solution for 18 h following Alloxan injection to prevent initial drug-induced hypoglycaemic mortality. After 18h of fasting, the rats were injected intraperitonially with alloxan monohydrate, dissolved in sterile normal saline at a dose of 100 mg/kg. Rats were acclimatized for a period of 7 days. After 7 days of Alloxan injection, animals with fasting blood glucose above 200 mg/dl were considered as diabetic and included in the study. The animals were randomly assigned into 5 groups of 30 animals each and received the following treatments:
Group- I: Normal healthy control (N=6) treated with distilled water.
Group-II: Diabetic Control (N=24) randomly divided into 4 groups of 6 rats each (diabetes induced by Alloxan monohydrate of 130 mg/kg).
Group-III: Diabetic Control treated with standard drug (Gliclazide 50 mg/kg) was given daily using an intragastric tube for 28 days.
Group-IV: Diabetic Control treated with methanolic seed extract of E. robusta (50 mg/kg) was given daily using an intragastric tube for 28 days.
Group-V: Diabetic Control treated with methanolic seed extract of E. robusta (100 mg/kg) was given daily using an intragastric tube for 28 days.
Body weights and blood glucose analysis was done weekly on overnight fasted animals. At the end of the experimental period, the animals were fasted overnight and blood was collected for various biochemical estimations. The animals were sacrificed by cervical decapitation. The liver, kidney and pancreas were dissected out, immediately rinsed in ice cold saline; a portion of the organs were fixed in 10% neutral buffered formalin for histopathological analysis and remaining portion were stored for further biochemical estimations.
Collection of blood samples from rats
Following an overnight fasting for 18 h after 7 days of treatment, the animals were anaesthetized using chloroform and sacrificed. Blood was collected by cardiac puncture to quantify fasting blood glucose using Accu-Chek glucometer. Serum was separated by centrifugation at 2000 rpm for 20 minutes; blood glucose levels were measured on 0th,7th,14th,21st,and 28th day; and blood glucose was estimated by GOD-POD enzymatic method [23].
Histopathological studies
The pancreas, liver and kidney were instantly dissected out, excised and rinsed in ice cold saline solution [24]. The tissues were 10% formalin was used as a fixative and paraffin wax was used for embedding the tissues. Paraffin section were taken (5µm thick) and stained with Haematoxylin and Eosin (H & E) for histopathological examination. After dewaxing, all slides were examined under a light microscope for pathological studies.
Statistical analysis
Statistical analysis was performed by one-way Analysis of Variance (ANOVA) followed by Dunnett’s multiple test of comparison using Graph Pad Prism (Version-5) software and expressed as Mean ± S.E.M. A value of p < 0.05 was considered statistically significant. In the oral glucose tolerance test, the glucose tolerance of seeds extract of E. robusta treatment significantly increased (p < 0.05) compared with the vehicle control.
Results And Discussion
Anti-diabeticactivity
Effect of seeds extract of E. robusta on fasting blood glucose in Alloxan-induced diabetic rats
Oral glucose tolerance test is used to determine the altered carbohydrate metabolism during post glucose administration [25]. Alloxan-induced diabetic rats showed marked reduction in their body weights when compared to normal rats. The decrease in body weight could be due to poor glycemic control and excessive catabolism of structural proteins to provide amino acids for gluconeogenesis during insulin deficiency [26]. The methanolic seed extract of E.robusta dissolved in 1% tween 80 with normal saline was administrated orally to diabetic rats at dose of 50 and 100 mg/kg body weight to evaluate the antidiabetic activity. Both the doses reduced the blood glucose level on 0th,7th,14th,21stand 28th day, when compared with standard control. The percentage of glucose reduction was observed at dose 50 mg/kg body weight was (41.94%) and 100 mg/kg body weight (53.84%) on 28 day. Oral administration of methanolic seeds extract of E.robusta for 28 days showed an improvement in body weight in comparison to diabetic control and rats treated with gliclazide (Figure 1 & table 1). The higher body weight of seeds extract of E.robusta treated rats might be due to their improved glycemic control.
|
Blood Glucose (mg/dl) |
|||||
|
Groups |
Day-0 (Mean±SD) |
Day-7 (Mean±SD) |
Day-14 (Mean±SD) |
Day-21 (Mean ± SD) |
Day-28 (Mean±SD) |
|
I: Normal control |
90.50±5.577 |
94.33±7.815 |
91.67±6.212 |
90.17±3.656 |
91.33±6.314 |
|
II: Diabetic control |
352.3±6.121 |
355.5±6.058 |
358.3±4.412 |
362.2±5.811 |
371.7±4.033 |
|
III: Gliclazide (50mg/kg) |
346.3±6.947 |
272.3±7.005 |
197.8±5.981 |
152.8±7.468 |
131.7±3.559 |
|
IV: E.robusta Extract (50mg/kg) |
351.7±5.317 |
331.3±7.118 |
270.7±7.118 |
243.7±3.559 |
204.2±4.021 |
|
V: E.robusta Extract(100mg/kg) |
347.7±6.186 |
273.5±7.662 |
219.8±6.014 |
178.0±6.066 |
160.5±6.025 |
Table 1: Effect of methanolic seed extract of E.robusta on blood glucose (mg/dL) level in Alloxan induced diabetic rats [values are mean ± SD from 6 animals in each group, P values <0.05; when compared with normal control group, diabetic control group]
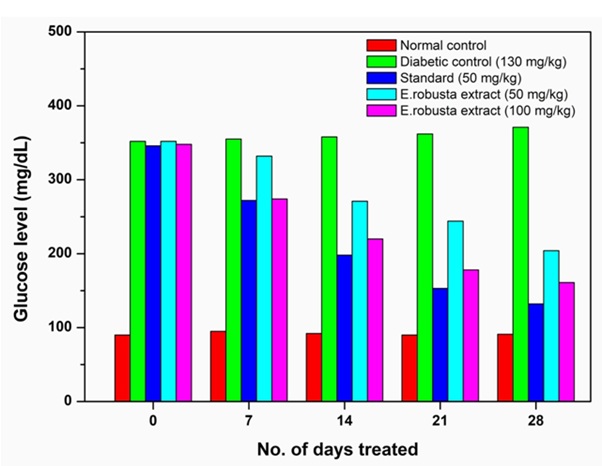 Figure 1: The variation of glucose level in mg/dL with various groups (I-V) from 0 day to 28 day in gliclazide-induced diabetic rats
Figure 1: The variation of glucose level in mg/dL with various groups (I-V) from 0 day to 28 day in gliclazide-induced diabetic rats
Effect of seeds extract of E. robusta on body weight in Alloxan-induced diabetic rats
Diabetes is also characterized by loss of body weight. Administration of methonolic seed extract of E.robusta at the dose 50 mg/kg and 100 mg/kg body weight in Group-IV and Group-V showed increased the body weight of 3.15% and 5.44%, respectively, and standard control 7.13% in (Group-III) showed significant increase (P<0.01) in the body weight in comparison to diabetic control showing significant decrease in the body weight (Figure 2 & table 2). Table 2 represents the changes in body weight in normal and experimental diabetic rats. Gliclazide treated diabetic rats showed significant (p < 0.001) decrease in the body weight as compared to normal animals. The diabetic control group continues to lose body weight till the end of the study, while administration of methonolic seed extract of E.robusta at both doses (50 and 100 mg/kg) showed significant improvement in body weight on days 14 and 21 when compared to diabetic control rats on respective days.
|
Body Weight |
||
|
Treatment Groups |
Initial body weight (Mean ± SD) |
Final body weight (Mean ± SD) |
|
Group-I: Normal Control |
160.7 ± 1.751 |
173.7 ± 2.066 |
|
Group-I: Diabetic control |
171.4 ±2.757 |
168.3 ±3.502 |
|
Group-III: Standard control(Gliclazide 50mg/kg) |
168.2 ±1.329 |
180.2 ± 2.944 |
|
Group-IV: E.robusta seed extract (T1) 50 mg/kg body weight |
165.0 ± 1.472 |
170.2 ± 2.858 |
|
Group-V: E.robusta seed extract (T2) 100 mg/kg body weight |
176.2 ± 1.862 |
185.8 ± 2.639 |
Table 2: Effect of body weight using methanolic seeds extract of E. robusta in control and diabetic induced rats after 28 day
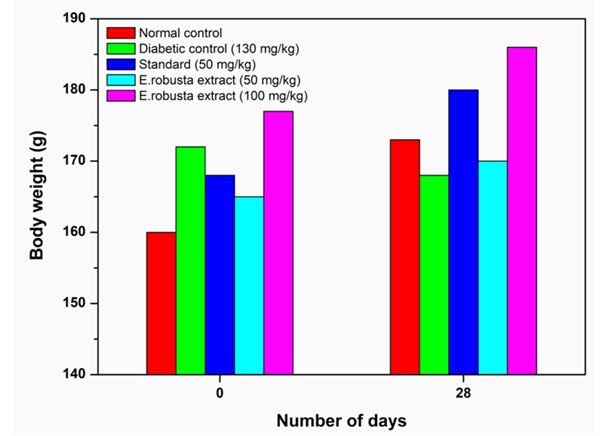 Figure 2: The variation of body weight using Methanolic seed extract of E.robustain 0 and 28 day with various groups (I-V) in gliclazide-induced diabetic rats.
Figure 2: The variation of body weight using Methanolic seed extract of E.robustain 0 and 28 day with various groups (I-V) in gliclazide-induced diabetic rats.
Oral administration of glucose (2 g/kg body weight) produced significant changes in blood glucose level in normal rats. The Peak increase in mean plasma glucose at 60 min in normal control, it was 176.0 mg/dL. Gliclazide treated animals has shown only 96.00 mg/dL, seed extract at the dose 50 mg/kg is 168.2 mg/dL and 100 mg/kg is 162.8 mg/dL, significantly shown plasma glucose at peak. In additional estimation of integrated AUC glucose Standard control has shown 36.53% (925.3 ± 11.95), seed extract at the dose 50mg/kg has shown 41.143% (1306 ± 14.89), at the dose 100 mg/kg has shown 31.41% (1216 ± 18.30), the total AUC compared with standard control (Table 3 & Figure 3). In correlation with the present study earlier E.ribes seed extract was found to have antidiabetic activity at the doses of 100 mg/kg and 200 mg/kg body weight [27]. Similarly antidiabetic activity of plant extract has been reported in many plants like Berberis aristata [28], Pterocarpus marsupium [29], and Allium sativum [30].
|
Treatment groups |
Blood Glucose Tolerance test |
|||
|
0 min (Mean ± SD) |
60 min (Mean ± SD) |
120 min (Mean ± SD) |
180 min (Mean ± SD) |
|
|
Normal control (Group I) |
94.33 ± 3.386 |
176.0 ± 2.608 |
144.8 ± 3.656 |
138.8 ± 2.483 |
|
Standard control (Group II) (Gliclazide 50mg/kg) |
91.17 ± 1.941 |
96.00 ± 2.828 |
92.33 ± 2.160 |
87.33 ± 2.658 |
|
E.robusta seed extract (T1) (Group III) 50 mg/kg body weight |
92.83 ± 2.483 |
168.2 ± 2.563 |
118.7 ± 4.033 |
116.8 ± 3.061 |
|
E.robusta seed extract (T2) (Group IV), 100 mg/kg body weight |
90.67 ± 3.986 |
162.8 ± 2.787 |
106.0 ± 3.578 |
101.5 ± 3.107 |
Table 3: Effect of Methanolic seed extracts of E.robusta with respect to time on oral glucose tolerance test with various groups (I-V) in gliclazide-induced diabetic rats.
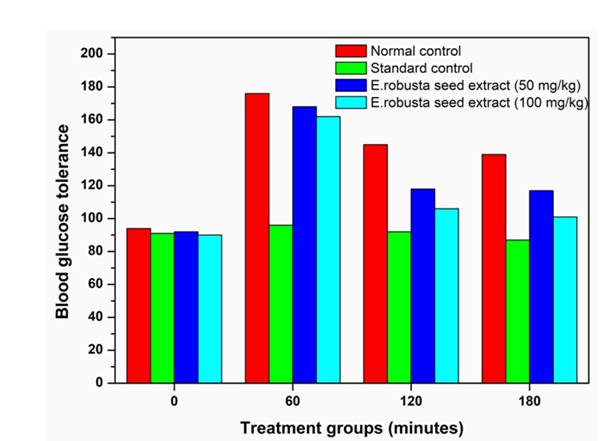 Figure 3: The variation of timeusing Methanolic seed extracts of E.robusta with various groups (I-V) in gliclazide-induced diabetic rats.
Figure 3: The variation of timeusing Methanolic seed extracts of E.robusta with various groups (I-V) in gliclazide-induced diabetic rats.
Alpha-glucosidase inhibition activity
Alpha-glucosidase inhibitory activity was determined by measuring the release of p-nitrophenyl-α-D-glucopyranoside (pNPG). The released p-nitrophenyl yields a yellow colour when the stopping reagent, glycine (pH = 10) is added. Alpha-glucosidase inhibitory activity of methanolic seed extract of E.robusta was analyzed and summarized in (Table 4 & Figure 4). Alpha-glucosidase inhibitory activity of methanolic seed extract of E.robusta was plotted as a function of concentration in comparison with acarbose was shown in Fig. 4. In this method starch was used as a substrate, which was converted by alpha-glucosidase into maltose which in turn reacted with 3,5-dinitrosalicylate to form colored product (α max 540 nm).The seed extract exhibited alpha-glucosidase inhibitory activity at all concentrations. Alpha-glucosidase inhibitory activity was increase with the increase concentration of the seed extract (500-31.25 µg/ml). The percentage of inhibitory activity of the E.robusta seed extract increased from 42.43 to 83.71%. Among all, maximum alpha-glucosidase inhibitory (%) was observed at 500 µg/ml concentration of seed extract with IC50 value 82.09 µg/ml. The standard acarbose showed % antioxidant activity with IC50 value of 30.61 µg/ml concentration.
|
Alpha - Glucosidase Inhibition |
||||
|
Concentration (µg/ml) |
Standard carbose inhibition, % |
Standard IC 50 values, µg/mL |
E.robusta seed extract-alpha glucocidase inhibition, % |
Seed extract IC 50 values, µg/mL |
|
500 |
92.38 |
30.61 µg/mL |
83.71 |
82.09 µg/ml
|
|
250 |
85.21 |
75.89 |
||
|
125 |
70.32 |
61.32 |
||
|
61.25 |
65.71 |
53.09 |
||
|
31.25 |
48.23 |
42.43 |
||
Table 4: Alpha-glucosidase inhibitory activities of methanolic seed extracts of E.robusta with standard and its IC50 values.
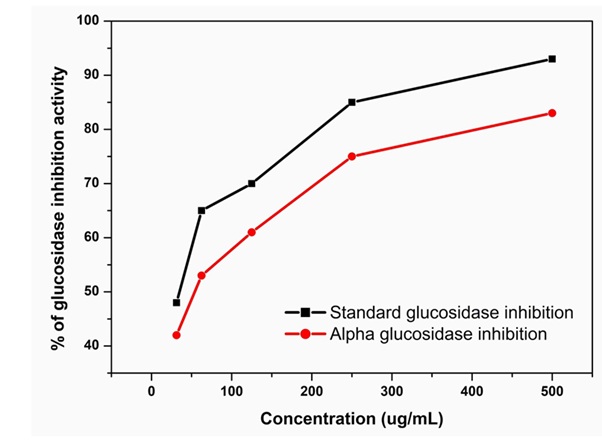 Figure 4: The Alpha-glucosidase inhibitory activities with the variation of concentration (µg/mL) using Methanolic seed extract of E.robusta.
Figure 4: The Alpha-glucosidase inhibitory activities with the variation of concentration (µg/mL) using Methanolic seed extract of E.robusta.
Effect of seeds extract of E. robustaon serum lipids in Alloxan-induced diabetic rats
The diabetic rats showed significant (p < 0.001) increase in the levels of serum TC, TG, VLDL-C, LDL-C and decreased HDL-C levels (Figure 5) (Table 5 & Table 6). Treatment with methanolic seed extract of E.robusta resulted in dose dependent reduction in TC, TG, VLDL-C and LDL-C compared to those in untreated diabetic rats while serum HDL-C levels were significantly (p < 0.01) increased in treated diabetic rats.
|
Treatment Groups |
Total cholesterol level (mg/dL) (Mean ± SD) |
Total Triglyceride level (mg/dL) (Mean ± SD) |
||
|
0-day (Mean ± SD) |
30-day (Mean ± SD) |
0-day (Mean ± SD) |
30-day (Mean ± SD) |
|
|
Normal Control |
80.50 ± 1.761 |
81.00 ± 2.280 |
81.33 ± 2.503 |
80.50 ± 3.834 |
|
Diabetic Control |
82.67 ± 3.559 |
130.2 ± 3.430 |
84.50 ±1.871 |
130.5 ± 2.881 |
|
Standard Control (Gliclazide, 50 mg/kg) |
81.17 ± 1.169 |
110.5 ± 2.168 |
82.83 ± 2.639 |
99.50 ±1.871 |
|
E.robusta seed extract (T1), 50 mg/kg bodyweight |
84.50 ± 1.643 |
126.0± 2.608 |
83.17 ± 2.639
|
128.2 ± 3.920
|
|
E.robusta seed extract (T2), 100 mg/kg body weight |
83.17 ± 2.858 |
116.3 ± 3.141 |
82.00 ± 1.789 |
108.7 ± 2.582 |
Table 5: Effect of methonolic seed extract of E.robusta on Total Cholesterol (TC) and Triglyceride (TG) Levels.
|
Treatment groups |
HDL (Mean ± SD) |
LDL (Mean ± SD) |
VLDL (Mean ± SD) |
|||
|
0-day |
30-day |
0-day |
30-day |
0-day |
30-day |
|
|
Normal control |
45.17 ± 2.137 |
45.17 ± 1.722 |
16.83 ± 2.787 |
19.73 ± 2.787 |
16.26 ± 2.805 |
16.1 ± 1.049 |
|
Diabetic control |
42.50 ± 2.345 |
30.00 ±3.688 |
21.17 ± 2.994 |
74.1 ± 3.488 |
16.9 ± 2.401 |
26.1 ± 3.141 |
|
Standard control (Gliclazide 50 mg/kg) |
43.50 ± 1.517 |
40.67 ±1.506 |
18.50 ± 1.871 |
49.93 ± 2.582 |
16.56 ± 2.251 |
19.9 ± 1.862 |
|
E.robusta seed extract (T1), 50 mg/kg bodyweight |
41.00 ± 2.000 |
33.17 ± 3.869 |
24.33 ± 2.160 |
67.73 ± 2.366 |
16.63 ± 2.366 |
25.6 ± 1.378 |
|
E.robusta seed extract (T2), 100 mg/kg bodyweight |
40.17 ± 1.329 |
35.67 ± 1.751 |
23.17 ± 1.329 |
58.63 ± 1.941 |
16.4 ± 1.871 |
21.7 ± 1.414 |
Table 6: Effect of methonolic seed extract of E.robusta on HDL, LDL, and VLDL Levels, n=6; Data expressed as mean ± SD.E valuation by One way Analysis of variance (Anova) Followed by post-hoctukey’s multiple comparison test; P < 0.05 as compared to diabetic control.
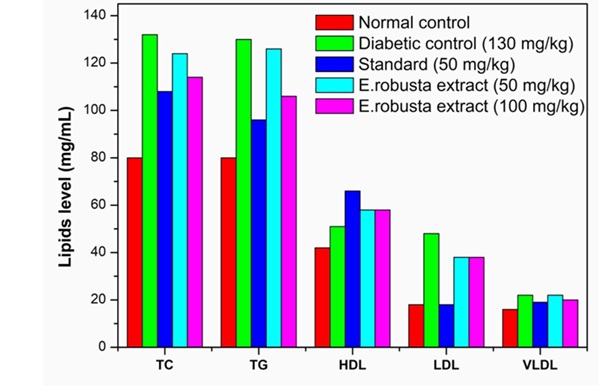 Figure 5: Effect of Methanolic seed extract of E.robustaon TC, TG, HDL, LDL, and VLDL.
Figure 5: Effect of Methanolic seed extract of E.robustaon TC, TG, HDL, LDL, and VLDL.
Histopathological activity of Pancreas in experimental rats
Histopathological studies (Figure 6) were carried out, in which normal healthy control group rats showed normal acini (Group-I), Diabetic control group rats showed extensive damage of the islet of langerhans of pancreas (Group-II), Gliclazide (50 mg/kg body weight) treated diabetic rats showed regeneration of β-cells (Group-III), seeds extract of E.robusta (50 mg/kg body weight) treated diabetic rats showed restoration of pancreatic β-cell (Group-IV) and seeds extract of E.robusta (100 mg/kg body weight) treated diabetic rats showed abundant regeneration of pancreatic β-cell also enlarged size of β-cells (Group-V). The histopathological studies have shown regeneration of β-cells by gliclazide (Group-III) in alloxan induced diabetic rats. Comparable β-cell regeneration was also observed in methanolic seed extract of E.robusta at the doses of 50 mg/kg body weight and 100 mg/kg body weight (Group-IV & Group-V) after 28 days of treatment. Similar studies of regeneration of pancreatic β-cells were observed by Gymnema sylvestre [31], curcumin longa [32] and Catharanthus roseus [33].
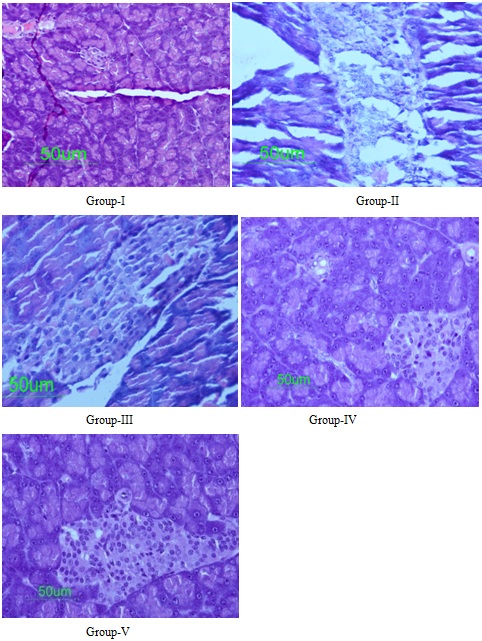 Figure 6: Histology of pancreas in experimental rats after 21 days of treatment (H & E, 50×): Group-I: Normal healthy control group; Group-II: Diabetic control group; Group-III: Standard group; Group-IV: Methanolic seed extract E.robusta (50 mg/kg); and Group-V: Methanolic seed extract of E.robusta (100 mg/kg)
Figure 6: Histology of pancreas in experimental rats after 21 days of treatment (H & E, 50×): Group-I: Normal healthy control group; Group-II: Diabetic control group; Group-III: Standard group; Group-IV: Methanolic seed extract E.robusta (50 mg/kg); and Group-V: Methanolic seed extract of E.robusta (100 mg/kg)
Conclusion
In conclusion, the antidiabetic activity and Histopathological study of pancreas using methanolic seed extract of E.robusta with various groups (Group-I to Group-V). It also reveals the comparison study of various weight doses of seed extract of E.robusta with standard drug gliclazide in the antidiabetic activity, blood glucose level and histopathological studies. It suggested that the methanolic seed extract of E.robusta at dose (100 mg/kg body weight, Group-V) exhibited significant anti diabetic activity than at dose (50 mg/kg body weight, Group-IV) in alloxan induced diabetic rats. E.robusta seed extract at dose (100 mg/kg body weight, Group-IV) has shown significant glucose lowering effect and the percentage of reduction (53.84%), where as the percentage of reduction in glucose levels foe standard gliclazide drug was (61.97%). The percentage of reduction for Group-IV was almost comparable to Group-III (standard). At the end of the study the Histopathological examination of pancreatic tissues shows β-cell regeneration which could be the reason for its reduction of glucose levels. These studies clearly showed that E.robusta seed extract has an excellent anti diabetic potential and can be used for the development of herbal formulations for control of diabetes mellitus and its clinical complication. Further studies on toxicity, lipid lowering properties and identification of bioactive principal of E.robusta seed extract will be explored in future work.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Author Contributions
Sharada seekonda was performed experimental work and drafted the manuscript. A. Roja Rani conceived the work and gave valuable insights.
Acknowledgment
Ms. Sharada seekonda sincerely acknowledge to the UGC-BSR scheme at Department of Genetics, Osmania University, Hyderabad for financial support.
References
- Joshi SR (2005) Management of Obese Indian Patient. Indian journal of Obesity 1: 11-20.
- Varghese R, Thomas B, hail MA, Rauf A, Sadi MA, et al. (2012) the prevalence, Risk Factor, Maternal and Fetal outcomes in Gestational Diabetic Mellitus. Int J drug Dev & Res 4: 356-368
- Ghoul JE, Smiri M, Ghrab S, Boughattas NA, Ben-Attia M (2012) Antihyperglycemic, antihyperlipidemic and antioxidant activities of traditional aqueous extract of Zygophyllum album in streptozotocin diabetic mice. Pathophysiology 19: 35-42.
- Islam MS (2011) Effects of the aqueous extract of white tea (Camellia sinensis) in a streptozotocin-induced diabetes model of rats. Phytomedicine 19: 25-31
- Rother KI (2007) Diabetes treatment-bridging the divide. N Engl J Med 356: 1499-1501.
- Cline GW, Petersen KF, Krssak M, Shen J, Hundal RS, et al. (1999) Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N Engl J Med 341: 240-246.
- Davidson S, Haslett C (1999) Davidson’s principles and practice of medicine (18th edn). Churchill Livingstone, London, United Kingdom. Pg no: 808.
- Paneni F, Beckman JA, Creager MA, Cosentino F (2013) “Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I,” European heart Journal 34: 2436-2443.
- Fowler MJ (2007) Diabetes treatment, part 2: oral agents for glycemic management. Clin Diabetes 25: 131-134.
- Markussen J (1999) New insulins: Types and actions. In: Turtle JR, KanekoT, Osato S (eds.). Diabetes in the new millennium. Sydney: The Endocrinology and Diabetes Research Foundation of the University of Sydney, Australia. Pg no: 251-264.
- Scott NA, Jennings PE, Brown J, Belch JJ (1991) Gliclazide: a general free radical scavenger. European Journal of Pharmacology: Molecular Pharmacology 208: 175-177.
- Pitocco D, Martini F, Zaccardi F, Ghirlanda G (2012) Antioxidants, healthydiet, and diabetes: oxidative stress and nutrition in diabetes. In: BagchiD, Sreejayan N (eds.). Nutritional and therapeutic interventions for diabetes and metabolic syndrome. Academic Press, San Diego, California. Pg no: 299-313.
- Bent S (2008) Herbal medicine in the United States: review of efficacy, safety, and regulation: grand rounds at University of California, San Francisco Medical Center. J Gen Intern Med 23: 854-859.
- Grijesh KM, Mishra PK, Prakash V (2009) Antidiabetic and hypolipidemic Activity of Gymnemasylvestre in Alloxan Induced Diabetic Rats, Global Journal of Biotechnology & Biochemistry 4: 37-42.
- Sharan SB, Mondal P, Zaman K, Junajo JA, Verma VK, et al. (2013) In vivo Anti-Diabetic Activity of the Methanolic and Aqueous Bark Extract of the Plant Embelicaofficinalis Gaertn. Academic Journal of Plant sciences 6: 64-68.
- Fatima S, Qureshi S, Pramodini GN, Rathour MS, Sharma V, et al. (2013) Antidiabetic activity of Polyherbal formulation containing Momordicacharantia, Caricapapaya, Trigonella fornum and Curcuma Longa (MCTC) in Alloxan induced Diabetic rats. International journal of Biological & pharmaceutical Research 4: 340-348.
- Mittal R, Ahmad M, Singh G (2005) Citation mapping of published literature on Embelia ribes, Annals of Library and Information Studies 52: 154-159.
- Kannur DM, Hukkeri VI, Akki KS (2006) Antidiabetic activity of Caesalpinia bonducella seed extracts in rats, Fitoterapia 77: 546-549
- Nagmoti DM, Kothavade PS, Bulani VD, Gawali NB, Juvekar AR (2015) Antidiabetic and antihyperlipidemic activity of Pithecellobium dulce (Roxb.) Benth seeds extract in streptozotocin-induced diabetic rats, European Journal of Integrative Medicine 7: 263-273.
- Sasidharan N (2000) Medicinal plants of Kerala forests. Indian Journal of Arecanut, Spices and Medicinal Plants 2: 115-139
- Souravi K, Rajasekharan PE (2014) A Review on the pharmacology of Embelia ribes BURM. F. A threat end medicinal plants, International journal of Pharma and Bio Science 5: 443-456.
- Joshi R, kamat JP, Mukherjee T (2007) Free radical scavenging reaction and antioxidant activity of embelin: Biochemical and pulse radiolytic studies. Chemico-Biological Interaction 167: 125-34.
- Trinder PB (1969) Determination of blood glucose using an glucose oxidase peroxidise system with a non-carcinigenic chronogen, J.Clin.Patho l22: 158-162.
- Luna LG (1960) Manual of histologic staining methods of the Armed Forces Institute of Pathology. McGraw Hill Book Co, New York. Pg no: 125.
- Ceriello A (2005) Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes 54: 1-7.
- Kasetti RB, Maddirala DR, Vinay KK, Shaik SF, Ethamakula GTK, et al. (2010) Antihyperglycemic and antihyperlipidemic activities of methanol:water (4:1) fraction isolated from aqueous extract of Syzygium alternifolium seeds in streptozotocin induced diabetic rats. Food Chem Toxicol 48: 1078-1084.
- Bhsndari U, Ansari MN (2008) Antihypercaemic activity of aqueous extract of Embelia ribes Burm in streptozotocin-induced diabetic rats, Indian Journal of Experimental Biology 46: 607-613.
- Mishra P, Rani A, Mazumder MP (2010) Blood Glucose Lowering Potential of stem of Berber aristata DC in Alloxan–Induced Diabetic Rats, Iranian Journal of Pharmacology & Therapeutics, IJPT 9: 21-24.
- Maruthupandian A, MohanVR (2011) Antidiabetic, Antiyperlipidaemic and Antioxidant activity of Pterocarpous Marsupium Roxb. In alloxan induced diabetic rats. International Jounrnal of PharmaTech Research 3: 1681-1687.
- Tripathi P, Gupta PP, Lal VK (2013) Effect of Co-administration of Allium sativum extract and Metformin on blood glucose of Streptozotocin induced diabetic rats, J Intercult Ethnopharma col 2: 81-84.
- Shanmugasundaram KL, Gopinath KR, Shanumugasundaram, Rajendran VM (1990) possible regeneration of the islets of Langerhan in streptozotocin-diabetic rats given Gymnema sylvestre leaf extracts. Journal of Ethnopharmacology 30: 65-79.
- Hamid MA, Moustafa N (2013) protective effect of curcumin on histopathology and ultrastructure of pancreas in the alloxan treated rats for induction of diabetes. The Journal of Basic & Applied Zoology 66: 169-179.
- Natarajan A, Zameer KS, Ahmed K, Sundaresan S, Sivaraj A, et al. (2012) Effect of Aqueous Flower Extract of Catharanthus roseus on Alloxan induced Diabetes in Male Albino Rats, International Journal of Pharmaceutical Science and Drug Research 4: 150-153.
Citation: Seekondaa S, Ranib R (2020) Evaluation of Antidiabetic and Histopathological Activities of Embelia robusta Seeds Extract against Alloxan-Induced Diabetic Wistar Rats. J Diabetes Metab Disord 7: 037
Copyright: © 2020 Sharada Seekondaa, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

