
Microgravity Significantly Influences Neural Stem Cells Size and Numbers: Implications for Long-term Space Missions
*Corresponding Author(s):
Araceli Espinosa-JeffreyDepartment Of Psychiatry, Semel Institute For Neuroscience And Human Behavior At University Of California Los Angeles, Los Angeles, United States
Tel:+1 3108251747,
Email:aespinosa@mednet.ucla.edu
Abstract
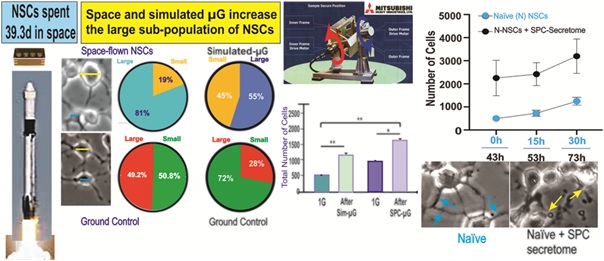
There are two approaches used to study the effects of microgravity on cells -- using simulated microgravity on Earth (sim-µG) or sending cells to space (SPC-µG). We have recently reported that human neural stem cells (NSCs) proliferated seven-times more while in space than Ground Control (GC) NSCs on Earth. Here, using time-lapse microscopy, we determined that in both sim-µG and SPC-µG there are two cell subpopulations distinguished by differences in somatic diameter. In the case of SPC-flown NSCs vs. GC, the proportion of 'large' diameter cells, classified as such when over 10 µm in diameter, was significantly greater at 81% of the total population measured, while GC cells exhibited a much smaller percentage of 'large' NSCs at 49.2%. After exposure to sim-µG, the percentage of ‘small’ NSCs with a cell diameter under 10 µm was 45%, while the number of NSCs exhibiting a large diameter increased to 55%. With respect to control NSCs maintained in 1G, most (72%) of these cells were ‘small’, while 28% of the NSCs were larger than 10 µm. Thus, the current study shows that SPC-µG exposure generated a greater proportion of 'large' NSCs than not only the GC cells, but also the sim-µG treated cells. Addition of SPC-NSCs secretome to naïve NSCs increased both proliferation and cell size. After 30h cells showed signs of unhealthy morphology, revealing a deleterious effect of SPC_NSC secretome.
Keywords
Cell diameter; Cell proliferation; Differentiation; Human neural stem cells; Intracranial hypertension; Microgravity; Long-term space exploration; Simulated microgravity
Graphical Abstract
Introduction
Human induced Pluripotent Stem Cells (hiPSCs) are generated by direct reprogramming of human somatic cells to a pluripotent stage through ectopic expression of specific transcription factors. These cells self-renew and possess the capacity to differentiate into any cell type of the human body (rev. de Souza et al., 2013). Their clinical applications or potential to study disease biology through modeling makes hiPSCs highly important. Moreover, they can also be used to test new drugs, and their potential toxicity [1].
Much is still unknown regarding the long-term effects of space microgravity (SPC-µG) on the CNS. One area of focus is the impact of SPC-µG on NSCs, which are still present and salient in the adult brain for their key roles in development, repair and maintaining homeostasis [2,3]. There is increasing evidence that spaceflight induces intracranial hypertension and visual impairment [4].
Microgravity has been previously reported to have the strongest effects on the sensorimotor, cerebellar, and vestibular areas of the brain [5]. Central venous and intercranial pressure are reduced from microgravity exposure, yet not to the extent observed in 90° seated upright posture on Earth. Instead, basal levels over 24h in microgravity are reported to remain slightly elevated, which may explain eye remodeling astronauts experience [6] as well as space flight-associated neuro-ocular syndrome (SANS) [7]. It has also been reported that microgravity induces apoptosis, alters the cytoskeleton, and affects differentiation, proliferation, and metabolic status at the cellular level [8].
As pluripotent stem cells, NSCs are the sole CNS cells with the ability to regenerate the main cell populations that form the CNS, i.e., astrocytes, oligodendrocytes, and neurons. Therefore, the influence of SPC-µG on NSCs will also have health impacts. We have previously reported that NSCs proliferated more while in space when compared to GC cultures [9]. In the present study using time-lapse microscopy, we sought to determine the effect of sim-µG and SPC-µG on post-flight NSCs proliferation and cell size. To our surprise, we found two cell sub-populations in the cultures of SPC-flown NSCs, distinguished by differences in cell diameter. Based on cell diameter, we also found two subpopulations within NSCs exposed to sim-µG NSCs when compared to SPC-NSCs. Insights on the effects of sim-µG on other human cell populations, have been reported including alterations on bone cell signaling and cartilage Extracellular Matrix (ECM) synthesis [10]. In conclusion, our current data shows increased proliferation after space flight and increased number of cells with a larger cell soma than GC cells. These two phenomena may contribute to intracranial hypertension in astronauts. Future work should analyze in depth microgravity-produced changes of NSCs at the epigenetic level.
Materials And Methods
Cells and culture system: hiPS-NSCs
A homogeneous population of NSCs was obtained from hiPSCs. The original cells, known as “CS83iCTR-33nxx” were derived from fibroblasts that were “reprogrammed” and provided to us by Cedars-Sinai Medical Center via a material transfer agreement. We then induced these hiPSCs to the neural phenotype using our STM medium. For detailed information on the culture medium and cell preparation see [11]. The main steps of the protocol are seen in figure 1.
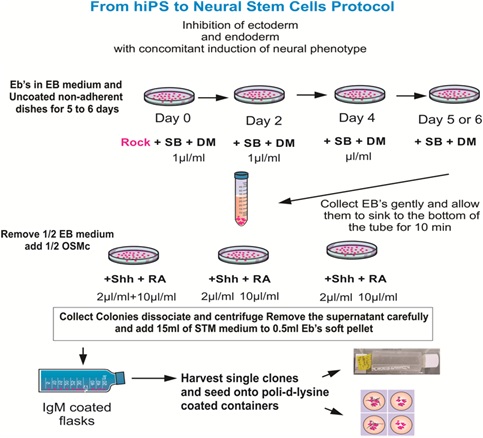 Figure 1: Ectoderm induction was performed by exposing hiPSCs to SB431542 (SB) and dorsomorphin (DM), which instruct ectoderm formation preferentially, with the concomitant inhibition of endoderm and mesoderm formation. All reagents were freshly prepared for optimal results. The substrate or coating of choice was IgM. Nonetheless, if the EBs have been exposed to 4°C while in suspension they would clump together, and the vast majority will not adhere to the IgM-coated surface. In this case, it is imperative to use Matrigel or poly-D-lysine (Figure modified from Espinosa et al., Current Protocols 2016).
Figure 1: Ectoderm induction was performed by exposing hiPSCs to SB431542 (SB) and dorsomorphin (DM), which instruct ectoderm formation preferentially, with the concomitant inhibition of endoderm and mesoderm formation. All reagents were freshly prepared for optimal results. The substrate or coating of choice was IgM. Nonetheless, if the EBs have been exposed to 4°C while in suspension they would clump together, and the vast majority will not adhere to the IgM-coated surface. In this case, it is imperative to use Matrigel or poly-D-lysine (Figure modified from Espinosa et al., Current Protocols 2016).
Naïve NSCs derived from human embryonic brain
Use of human embryonic NSCs was approved by the Office of the Human Subject Committee. Cultures of NSCs were prepared with fetal human tissue specimens donated by the Department of Pathology and Laboratory of Medicine at UCLA. Samples were de-identified in accordance with NIH guidelines. Anonymous, preserved specimens are donated for medical research purposes and are IRB exempt ( www.pathology.ucla.edu/TPCL.html ). NSCs were prepared and cultured in STM medium just as hiPS- derived NSCs, as previously reported [12].
Ground Control (GC) cells were grown in hardware and pre-seeded onto mesh carriers following the same procedure as the SPC cells. Type IV units were selected for the study due to their feature of refreshing culture medium using fresh medium from the second tank. The first tank contained medium with bromodeoxyuridine (BrdU), a synthetic “thymidine analog”, whereas the second did not. The culture medium was equilibrated overnight at 5% CO2 in the incubator before the flight implementation, where cells were maintained.
The SPC-flown NSCs were seeded onto mesh carriers and placed “free floating” using pre-equilibrated culture medium in the cell chamber of Automated Type IV units from Yuri (Meckenbeuren, Ger). The culture medium was pre-equilibrated at 5% CO2 because the Space Technology and Advanced Research System Experiment Facility-1 (STaARS EF-1) incubator does not provide CO2 capabilities. The Type IV units contained one Culture Chamber with a volume of 11.5ml ± 0.3ml. In addition, the units contained two Media Exchanges - Refreshment Medium and Fixative, as well as two Tanks with a volume of 11 ml ± 0.3 ml each. The hardware is flight-proven and consists of outer shell covers as well as the cell culture chamber and integrated tanks containing fresh culture media in the inner shell. The time required for automated media exchange is between 5 to 7 minutes. This hardware from Yuri (formerly known as Kiwi and, prior to it, Airbus) was leased by STaARS for this study for both GC and SPC conditions. The hardware-units containing the experiment can clip-in-place onto the STaARS EF-1 to electrically and mechanically interface with it (Figure 2).
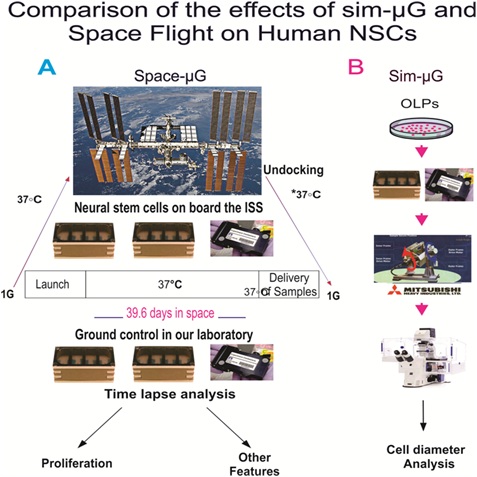 Figure 2: A) Synopsis of passive experiments. After seeding NSCs on floating mesh carriers that promote strong cell adhesion, 4 cell carriers of 2 x 2mm were placed in each well pre-launching and they spent a total time of 39.6 days in space. Upon splash-down they were transferred to the Long Beach (CA, USA) Airport and brought to the laboratory (UCLA) at 37°C. Upon arrival, cells were seeded onto flasks or flaskettes and allowed to recover for six hours in the incubator and subsequently they were placed in the imaging system. B) Cells were plated in the same type of units for the sim-µG study. All units, whether in space, sim-µG or GCs (1G) were maintained in the self-contained black cover [9].
Figure 2: A) Synopsis of passive experiments. After seeding NSCs on floating mesh carriers that promote strong cell adhesion, 4 cell carriers of 2 x 2mm were placed in each well pre-launching and they spent a total time of 39.6 days in space. Upon splash-down they were transferred to the Long Beach (CA, USA) Airport and brought to the laboratory (UCLA) at 37°C. Upon arrival, cells were seeded onto flasks or flaskettes and allowed to recover for six hours in the incubator and subsequently they were placed in the imaging system. B) Cells were plated in the same type of units for the sim-µG study. All units, whether in space, sim-µG or GCs (1G) were maintained in the self-contained black cover [9].
SPC-µG
Our cells were flown aboard SpaceX-16 to the International Space Station (ISS). Before returning to Earth, the cells remained onboard the ISS for 39.3 days. 2mm x 3mm mesh carriers were placed into cell chambers for the cells. The culture medium was equilibrated overnight at 5% CO2 in the incubator where cells were maintained before the flight implementation. As for the cell cultures that were sent into SPC-µG, NSCs were seeded onto mesh carriers and placed “free floating” in the cell culture chamber of Automated Type IV units from Yuri. This hardware is flight-proven and entails both Outer Shell covers and the cell culture chamber and integrated tanks containing the culture medium, or Inner Shell. This hardware from Yuri (formerly known as Kiwi, and prior to it, Airbus) was leased from NASA for the Bioscience-4 experiment and was used for both GC and in-flight cells. These hardware-units containing the experiment clip-in-place onto the Space Technology and Advanced Research System-1 Experiment Facility (STaARS-1 EF) and interface mechanically and electrically with it (Figure 2A). (For further details on the hardware see: https://www.yurigravity.com/our-service). After harvesting and replating, NSCs were allowed to recover for 6h following the space flight and transit. Subsequently, NSCs were placed in a time-lapse microscope system (Zeiss, Oberkochen, Germany). Images were acquired every 15 min for a longitudinal study.
Simulated microgravity
For the sim-µG portion of this study, we used the 3D-clinostat that was designed and produced by Mitsubishi Heavy Industry, as previously described [13]. The outline of operational principle is described together with its mechanical design as follows: rotation around two independent axes makes the direction of gravity vector to scan the whole steric angle. While simulated microgravity cannot be directly equated to SPC-µG, the Mitsubishi 3D-clinostat had a minimal gravity force that led to similar results in terms of enhanced proliferation. This 3D-clinostat was used to simulate microgravity and is referred to as the sim-µG condition for NSCs for the remainder of the paper (Figure 2A). The sim-µG NSCs were exposed to sim-µG for 72h.
Time-lapse microscopy
Time-lapse imaging for cell proliferation studies were conducted with the ZEISS Axio Observer 7 fully motorized inverted research microscope equipped with Definite Focus 2, ZEISS Axiocam 506 monochrome camera with ZEISS ZEN software, and ZEISS Full Incubation XL chamber for Temperature and CO2 control with a motorized scanning stage (Figure 2B). Live cell imaging equipment was essential as we wanted to capture the behavior of cells as they were adapting to Earth gravity. The controlled temperature and gas environment were equivalent to that of NSCs maintained in our cell culture incubator. Images were collected every 15 minutes at each of 6 to 8 xyz positions for a 72-hour duration.
Statistics
Data are presented as mean ± SD and statistical analyses were performed using One-Way ANOVA, followed by Tukey post-hoc test in which p< 0.05 was defined as statistically significant. For the analysis of naïve cells, a two-way ANOVA was performed, followed by Sidak’s multiple comparisons [14] test, in which *p< 0.05 was defined as statistically significant. For proportion statistical comparisons, the Chi-square test was used.
Results
Mitotic activity of NSCs following space flight or sim-µG
In all 1G control and SPC-flown NSC cultures, proliferation was observed, increasing the total cell count during the 72h during which continuous live imaging was performed post-flight. Proliferation was significantly greater after Sim-µG and Space-Microgravity compared to controls. However, by 30h all differences between groups became non-significant. We can conclude these SPC-flown NSCs and their progenies preserve their proliferative capacity for 15h, but not 30h. This indicated NSCs are in good health as this capacity is a feature proper to NSCs. Nonetheless, with increasing time on Earth, the rate of proliferation decreased with time tending to normalize starting at 30h where differences were not significant (Figure 3).
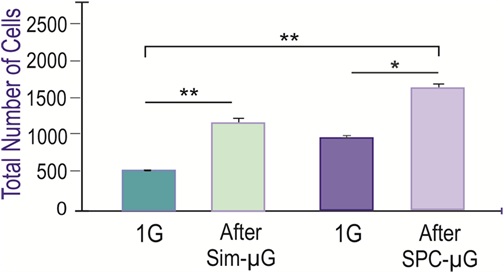 Figure 3: Similar Influence of Simulated-µG and Space-Microgravity on Cell Proliferation. Comparison of NSCs proliferation after exposure to simulated microgravity or spaceflight. The figure depicts cell count values from six fields chosen randomly. The NSCs tested were cultured either in simulated microgravity during 72h and the corresponding 1G control in the same incubator (green bars), or after spaceflight and their corresponding GC (purple bars). Data Analysis was performed with One-way ANOVA followed by Tukey multiple comparisons test in which *p < 0.05 was defined as statistically significant; **p < 0.01. Data represent the mean of three separate cultures ± SD.
Figure 3: Similar Influence of Simulated-µG and Space-Microgravity on Cell Proliferation. Comparison of NSCs proliferation after exposure to simulated microgravity or spaceflight. The figure depicts cell count values from six fields chosen randomly. The NSCs tested were cultured either in simulated microgravity during 72h and the corresponding 1G control in the same incubator (green bars), or after spaceflight and their corresponding GC (purple bars). Data Analysis was performed with One-way ANOVA followed by Tukey multiple comparisons test in which *p < 0.05 was defined as statistically significant; **p < 0.01. Data represent the mean of three separate cultures ± SD.
As described in methods, a 3D-clinostat was used in the laboratory to simulate microgravity. The corresponding 1G control was kept in the same incubator just without the rotation. We compared the effects of sim-µG to SPC-µG 24h after removal from the 3D-clinostat or after space flight. We found a higher number of NSCs in cultures exposure to the sim-µG. Specifically, there were more than twice as many NSCs in sim-µG when compared to GCs. Furthermore, the number of NSCs after the space flight was also increased with respect to GC samples indicating that after space flight NSCs continued proliferating more, as if they ‘remembered’ having been in space (Figure 3). The increased proliferation of these sim-µG cells shows that in terms of proliferative ability, sim-µG and SPC-µG conditions produce comparable results.
These experiments were performed on Earth either in Earth gravity or using sim-µG (while on Earth). Thus, to differentiate them from the experiments where NSCs grew either on Earth as GC or while in SPC-flight we called control cells 1G.
Space flight influences NSCs subpopulations
NSCs were removed from the flight hardware and plated onto poly-d-lysine coated flaskettes. Cultures were kept in the incubator at 37°C and 5% CO2 to recover from space travel and splashdown. Six hours later, NSCs were placed in a time-lapse microscope equipped with temperature and CO2 control. While not every cell was larger, measurements yielded an increased average diameter. The diameter of NSCs was measured 24h after seeded. Random samples of 40 cells were selected, excluding outliers (as determined by Grubbs test), and subsequently categorized based on their properties. Cell diameter was measured using ZEISS ZEN Blue microscopy software in µm. We found two sub-populations of cells that we divided into two categories, the small population measuring between 7 µm and 10 µm, and the large population ranging from over 10 µm to 25 µm in diameter (Figure 4). These categories of small and large were determined based on the data, which on average split nicely into one of the two sizes. The SPC-flown NSCs were larger on average both as a whole and within the two subpopulations. For example, SPC-flown NSCs were, on average, 12.53 µm initially, and 12.6 µm at 24h, whereas GC cells measured on average 9 µm at 0h and 10.67 µm after 24h. These differences between control and SPC-flown NSCs were statistically significant. Moreover, the differences between the two time points were also significant within groups. As for the small diameter sub-population, we also saw differences in diameter, with the SPC-flown small diameter cells measuring 9.250 µm on average at 0h, while the GC NSCs at 0h were much smaller, 8.465 µm on average. This shows that whether we are looking across a 24h period or simply comparing the sub-populations at 0h after seeding, we can see an average larger cell size with the SPC-flown group (Figure 4). In agreement, we found a significant increase in the proportion (Figure 5) and relative distribution (Figure 6) of large SPC-flown NSCs compared with GC.
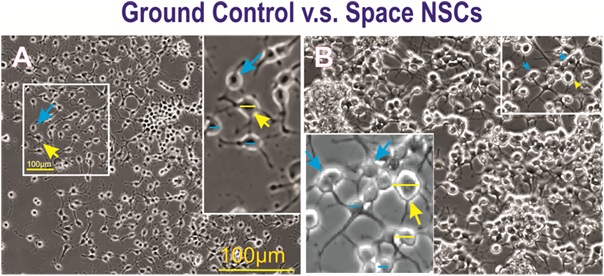 Figure 4: A) Field views of ground control NSCs grown on Earth gravity (GC) or B) NSCs after spaceflight.Examples of cell diameters of large (15µm or more indicated with yellow arrows) and small (equal orunder 10 µm, indicated with blue arrows) NSCs. Their diameter was measured using the Zen blue hardware. A) GC NSCs at 24h displayed a more homogeneous size. B) Cultures from SPC-flown NSCs 24h after platingshowed larger cells than control cultures. Insets show magnified views of the cells from the full frame images.
Figure 4: A) Field views of ground control NSCs grown on Earth gravity (GC) or B) NSCs after spaceflight.Examples of cell diameters of large (15µm or more indicated with yellow arrows) and small (equal orunder 10 µm, indicated with blue arrows) NSCs. Their diameter was measured using the Zen blue hardware. A) GC NSCs at 24h displayed a more homogeneous size. B) Cultures from SPC-flown NSCs 24h after platingshowed larger cells than control cultures. Insets show magnified views of the cells from the full frame images.
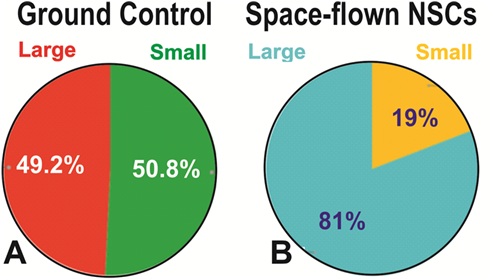 Figure 5: Proportion of small vs. large NSCs in GC and SPC-flown populations. Panel A shows that the small and large NSCs grown in Earth gravity were evenly distributed, large NSCs accounted for 49.2% and small NSCs represented 50.8% of the entire population. Panel B shows that the proportion of cells included in each group shifted after exposure to SPC-µG, where large cells represented 81% of the total number of cells while the percentage of small NSCs had decreased to 19% (p=0.00001, Chi-square). Results are from three separate experiments, a total of 1800 cells per condition were measured. The same data are reported as mean ± SD in figure 6.
Figure 5: Proportion of small vs. large NSCs in GC and SPC-flown populations. Panel A shows that the small and large NSCs grown in Earth gravity were evenly distributed, large NSCs accounted for 49.2% and small NSCs represented 50.8% of the entire population. Panel B shows that the proportion of cells included in each group shifted after exposure to SPC-µG, where large cells represented 81% of the total number of cells while the percentage of small NSCs had decreased to 19% (p=0.00001, Chi-square). Results are from three separate experiments, a total of 1800 cells per condition were measured. The same data are reported as mean ± SD in figure 6.
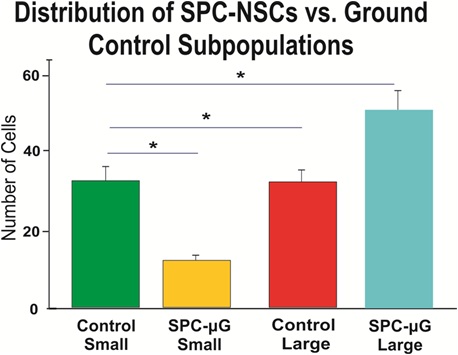 Figure 6: The bar graph shows the average size of 1G control NSCs was smaller for both classifications, small and large cell diameter. The average size of 1G small NSCs was 8.46 µm whereas for SPC NSCs the average small cell was 9.5 µm. Large SPC-treated NSCs averaged 16.76 µm, compared to the average large control NSC at 15 µm. Data analysis was performed with one-way ANOVA followed by a post-hoc Tukey HSD Test in which *p< 0.05 was defined as statistically significant. Results are from three separate experiments; a total of 1800 cells per condition were measured. Data are reported as mean ± SD (*p < 0.0001).
Figure 6: The bar graph shows the average size of 1G control NSCs was smaller for both classifications, small and large cell diameter. The average size of 1G small NSCs was 8.46 µm whereas for SPC NSCs the average small cell was 9.5 µm. Large SPC-treated NSCs averaged 16.76 µm, compared to the average large control NSC at 15 µm. Data analysis was performed with one-way ANOVA followed by a post-hoc Tukey HSD Test in which *p< 0.05 was defined as statistically significant. Results are from three separate experiments; a total of 1800 cells per condition were measured. Data are reported as mean ± SD (*p < 0.0001).
Characterization of NSCs grown in sim-µG showed a greater density and an apparent greater variety in cell size when compared to their 1G counterparts (Figure 7). The proportion of large cells was also increased under sim-µG as compared to the 1G control group, with 55% of the sim-µG cells qualifying as 'large' with diameters over 10 µG, and only 28% of the sample of 1G control NSCs qualifying as 'large' in diameter. However, overall, the size of the sim-µG treated NSCs was closer to that of the 1G cells than the SPC-flown NSCs (Figure 8).
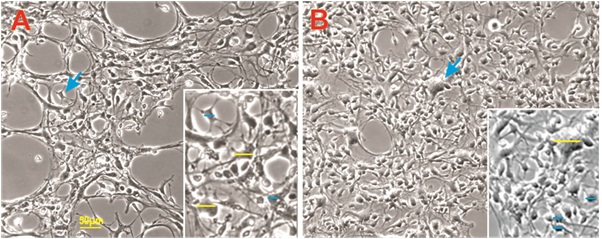 Figure 7: Examples of 1G (A) and sim-µG (B) NSCs at the same time point. Sim-µG treated NSCs exhibited higher rates of proliferation shown by greater cell density in the image. Small cells are designated in blue as equal or under 10 µm in diameter, and large cells are shown in yellow with diameters over 10 µm.
Figure 7: Examples of 1G (A) and sim-µG (B) NSCs at the same time point. Sim-µG treated NSCs exhibited higher rates of proliferation shown by greater cell density in the image. Small cells are designated in blue as equal or under 10 µm in diameter, and large cells are shown in yellow with diameters over 10 µm.
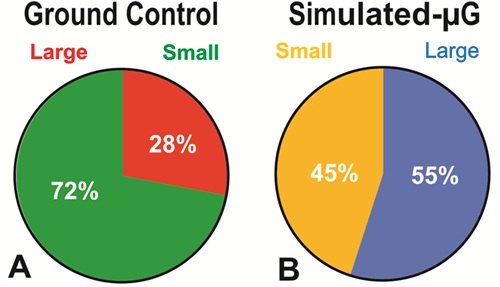 Fgure 8: Diameter Comparison between human NSCs Grown in sim-µG or Earth Gravity (1G). Cells were cultured in STMc medium for 24h and then photographed to perform measurements. A) The analysis revealed that in Earth gravity, there were more NSCs in the small-cell category accounting for 72% of the total population, while only 28% of the cells were large. B) The percentage of the two sub-populations of NSCs exposed to sim-µG had significantly changed from 28% to 55% with a concomitant reduction of the small population group from 72% to 45% (P=0.0001, Chi-square). Therefore, there was a 27% increase in the large cell group. Results are from three separate experiments, a total of 1800 cells per condition were measured. The same data are reported in figure 9 as mean ± SD.
Fgure 8: Diameter Comparison between human NSCs Grown in sim-µG or Earth Gravity (1G). Cells were cultured in STMc medium for 24h and then photographed to perform measurements. A) The analysis revealed that in Earth gravity, there were more NSCs in the small-cell category accounting for 72% of the total population, while only 28% of the cells were large. B) The percentage of the two sub-populations of NSCs exposed to sim-µG had significantly changed from 28% to 55% with a concomitant reduction of the small population group from 72% to 45% (P=0.0001, Chi-square). Therefore, there was a 27% increase in the large cell group. Results are from three separate experiments, a total of 1800 cells per condition were measured. The same data are reported in figure 9 as mean ± SD.
The results of figure 9 show that there is a higher proportion of NSCs with a smaller (under 10 µm) cell body diameter when cultured under GC conditions as compared to sim-µG (3-D-clinostat) NSCs cultured in the same incubator. Cell number and significance for this part of the study are shown.
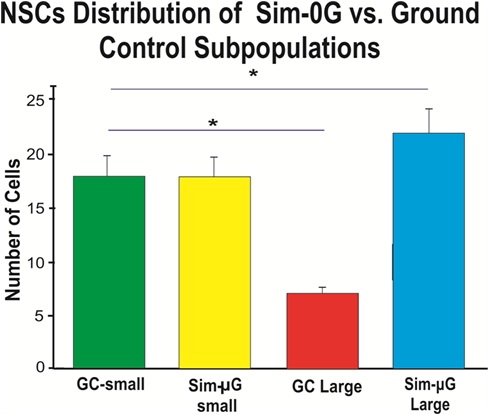 Figure 9: The bar graph shows that NSCs exposed to sim-µG exhibit more cells of a large size when compared to GC NSCs. While the population of small diameter cells is equal between GC and sim-µG treated populations, we found many more cells that classified as large diameter for sim-µG compared to the GC subpopulation. The average size of small GC NSCs was 7.27 µm, whereas for sim-µG treated NSCs, the average small cell was 7.49 µm. Large SPC-treated NSCs averaged 12.47 µm, compared to the average large control NSC at 11.33 µm. Results are from three separate experiments, a total of 1800 cells per condition were measured. Data are reported as mean ± SD (*p< 0.0001).
Figure 9: The bar graph shows that NSCs exposed to sim-µG exhibit more cells of a large size when compared to GC NSCs. While the population of small diameter cells is equal between GC and sim-µG treated populations, we found many more cells that classified as large diameter for sim-µG compared to the GC subpopulation. The average size of small GC NSCs was 7.27 µm, whereas for sim-µG treated NSCs, the average small cell was 7.49 µm. Large SPC-treated NSCs averaged 12.47 µm, compared to the average large control NSC at 11.33 µm. Results are from three separate experiments, a total of 1800 cells per condition were measured. Data are reported as mean ± SD (*p< 0.0001).
We have previously reported that the secretome from space-flown NSCs had effects on Earth-grown human NSCs increasing their proliferation (Shaka et al., 2021). In order to eliminate the possibility that our findings were inherent to hiPS-derived NSCs, we subsequently examined the effect of space-flown NSCs’ secretome on naïve embryonic-NSCs using time-lapse microscopy. These cells were seeded on poly-d-lysine coated flaskettes in STM medium. During the first 42 hours, NSCs were in their regular culture medium. They remained healthy and their cell processes interconnected with neighbor cells, for at least 43.33h, the duration of the experiment (Figure 10). Starting at hour 43, the same cells were treated with the secretome from space-flown NSCs in a ratio of 2:1 v/v stem medium: SPC-secretome respectively. The same naïve NSCs were treated with the NSCs-secretome starting at 43h. Initially, cells appeared healthy and were either bipolar or multipolar. Nonetheless as soon as 10h they had started to lose contact with their neighbors. Some had lost their cell processes and were surrounded by debris. Some cell processes were semi-truncated at this and subsequent time points (Figure 11). Moreover, after 24h in the secretome supplemented medium, the cell size of most NSCs tended to shift towards the large size, some measuring 15µm or larger in diameter. Cells also tended to form clusters as time in contact with SPC-secretome increased. Cells then started to die.
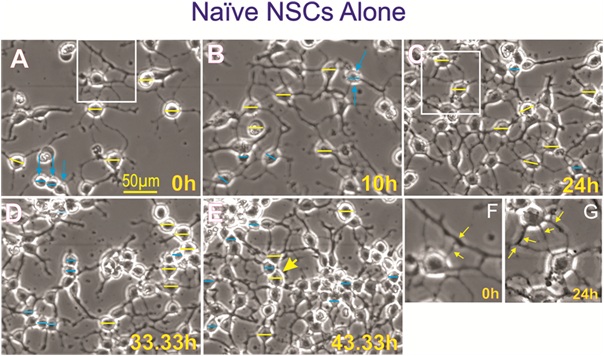 Figure 10: Control naïve neural stem cells. A) At the beginning of the experiment naïve NSCs in STM medium were mostly bipolar and some had three or more cell proesses. B to E) with time in culture, NSCs developed strong interconnecting branch-like processes. F) Higher magification view of a NSC with multiple processes. G) View of another cell with strong healty processes.
Figure 10: Control naïve neural stem cells. A) At the beginning of the experiment naïve NSCs in STM medium were mostly bipolar and some had three or more cell proesses. B to E) with time in culture, NSCs developed strong interconnecting branch-like processes. F) Higher magification view of a NSC with multiple processes. G) View of another cell with strong healty processes.
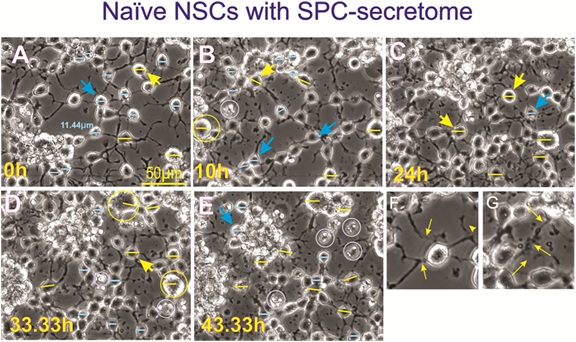 Figure 11: Naïve neural stem cells with secretome produced by SPC-flown NSCs. Naïve NSCs maintained in 1G were seeded and placed in the time-lapse system. Initially, imaging proceeded without adding the secretome from SPC-flown NSCs (Figure 7). Subsequently, starting at 43h cells were treated with the supernatant (secretome) of SPC-flown NSCs. A) View of these cells the moment the secretome was added to the cultures. Most if not all cells displayed three or more cell processes. B) 10h after adding the secretome a few cells started to look enlarged, examples are shown in yellow and where the bar measures 15µm. C) After 24h in space secretome some cells looked even larger, some had clustered and were losing cell processes. D) After 30h treatment, cells had enlarged considerably; examples are shown in yellow circles. E) Clustered and single cells looked unhealthy and extremely enlarged, some have started to die (white circles), and smaller cells formed rows. Many had lost their cell processes. F) Shows a cell at time 0 which had four strong cell processes that were in contact with surrounding cells. A thinner cell process also tended to contact another neighboring cell. G) Inset view of cells 24h after addition of SPC-secretome, these cells had started to lose their processes as shown by thin yellow arrows.
Figure 11: Naïve neural stem cells with secretome produced by SPC-flown NSCs. Naïve NSCs maintained in 1G were seeded and placed in the time-lapse system. Initially, imaging proceeded without adding the secretome from SPC-flown NSCs (Figure 7). Subsequently, starting at 43h cells were treated with the supernatant (secretome) of SPC-flown NSCs. A) View of these cells the moment the secretome was added to the cultures. Most if not all cells displayed three or more cell processes. B) 10h after adding the secretome a few cells started to look enlarged, examples are shown in yellow and where the bar measures 15µm. C) After 24h in space secretome some cells looked even larger, some had clustered and were losing cell processes. D) After 30h treatment, cells had enlarged considerably; examples are shown in yellow circles. E) Clustered and single cells looked unhealthy and extremely enlarged, some have started to die (white circles), and smaller cells formed rows. Many had lost their cell processes. F) Shows a cell at time 0 which had four strong cell processes that were in contact with surrounding cells. A thinner cell process also tended to contact another neighboring cell. G) Inset view of cells 24h after addition of SPC-secretome, these cells had started to lose their processes as shown by thin yellow arrows.
Cell measurements were performed at 0, 10h, 33h and 43h. We performed measurements of the two NSCs sub-populations on cells prior to adding the SPC-secretome. Naïve cells remained at their original size they displayed at the beginning of the study.
We have previously reported that the secretome from space-flown NSCs had effects on Earth-grown human NSCs increasing their proliferation (Shaka et al., 2021). In order to eliminate the possibility that our findings were inherent to hiPS-derived NSCs, we subsequently examined the effect of space-flown NSCs’ secretome on naïve embryonic-NSCs using time-lapse microscopy. These cells were seeded on poly-d-lysine coated flaskettes in STM medium. During the first 42 hours, NSCs were in their regular culture medium. They remained healthy and their cell processes interconnected with neighbor cells, for at least 43.33h, the duration of the experiment (Figure 10). Starting at hour 43, the same cells were treated with the secretome from space-flown NSCs in a ratio of 2:1 v/v stem medium: SPC-secretome respectively. The same naïve NSCs were treated with the NSCs-secretome starting at 43h. Initially, cells appeared healthy and were either bipolar or multipolar. Nonetheless as soon as 10h they had started to lose contact with their neighbors. Some had lost their cell processes and were surrounded by debris. Some cell processes were semi-truncated at this and subsequent time points (Figure 11). Moreover, after 24h in the secretome supplemented medium, the cell size of most NSCs tended to shift towards the large size, some measuring 15µm or larger in diameter. Cells also tended to form clusters as time in contact with SPC-secretome increased. Cells then started to die.
Cell measurements were performed at 0, 10h, 33h and 43h. We performed measurements of the two NSCs sub-populations on cells prior to adding the SPC-secretome. Naïve cells remained at their original size they displayed at the beginning of the study
Forty-three hours after these naïve NSCs were incubated in just culture medium, the same cells received NSCs-SPC secretome in a ratio 2:1 (2 part of culture medium and 1 part of SPC-NSCs secretome V:V. To differentiate naïve NSCs from “ground Control” from SPC-flown cells, we labeled naïve NSCs or N-NSCs + SPC-secretome. Two-way ANOVA with repeated measures on both 3 time points and 2 treatments (1G/1G NSC+SPC). After the addition of the secretome from space flown NSCs, the total number of cells increased by 4.6 times, 3 times, and 2.7 times for 43, 58, and 73 h, respectively (Figure 12).
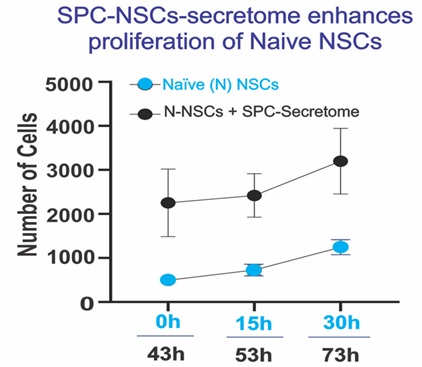 Figure 12: We observed that, as expected, Naïve 427 NSCs proliferated as a function of time (blue 428 dots). After adding the secretome from SPC-flown 429 NSCs, they proliferated even more, and these 430 differences were significant (black dots). Data 431 Analysis was performed with two-way ANOVA 432 followed by Sidak’s multiple comparisons test in 433 which *p< 0.05 was defined as statistically 434 significant. *p< 0.0026; **p< 0.0030; ***p< 0.0017. 435 Data represent three separate experiments 436 reported as ± SD.
Figure 12: We observed that, as expected, Naïve 427 NSCs proliferated as a function of time (blue 428 dots). After adding the secretome from SPC-flown 429 NSCs, they proliferated even more, and these 430 differences were significant (black dots). Data 431 Analysis was performed with two-way ANOVA 432 followed by Sidak’s multiple comparisons test in 433 which *p< 0.05 was defined as statistically 434 significant. *p< 0.0026; **p< 0.0030; ***p< 0.0017. 435 Data represent three separate experiments 436 reported as ± SD.
Discussion
Does simulated microgravity equate SPC-µG?
Here we report significant quantitative data showing that NSCs proliferated more in both, sim-µG and SPC-µG, when compared to NSCs grown in Earth gravity. This result confirms our previous work where we reported that sim-µG produced by a 3-D clinostat increased the proliferation rate of rodent and human NSCs and OLPs, respectively [9,12]. Therefore, in terms of cell proliferation we conclude that sim-µG and SPC-µG exert the same type of effect. Nonetheless, the influence of microgravity produced by clino-rotation on NSCs subpopulations was categorized by us into small and large subpopulations and yielded significant differences in terms of numbers across the two subpopulations, but not in terms of the type of effect. Our data is significant in showing that the clino-rotation method of µG simulation gives rise to an increased number of NSCs falling in the 'large' (55%) cell diameter group, compared to the 1G-control group which contained only 28% of large NSCs. Moreover, in NSCs exposed to SPC-µG, the proportion of 'large' diameter cells was significantly greater than control NSCs where 81% of the cells were in the large category with a diameter over 10µm. Therefore, it appears as if SPC-µG exerts a stronger effect on NSCs than clino-rotation. Nonetheless, it is important to note that NSCs were exposed to sim-µG for 72h while NSCs flown onto space were in microgravity for 39.3 days. More studies would be necessary to elucidate whether the length of exposure to either type of microgravity was a definitive determinant on the data generated as this cannot be affirmed at the present time. In terms of cell proliferation our data demonstrated that both sim-µG and SPC-µG increased NSCs proliferation when compared to their respective controls.
Identification of the origin of intracranial hypertension, a potential health risk in space exploration
Although at the present time with our data it is not possible to determine if these significant changes have major health implications in astronauts, we can affirm that at least for one cell population in the brain i.e., NSCs, there is a total increase of volume under SPC-µG, because more (31%) of the total NSCs population acquired the large-phenotype after space flight, resulting in a total of 81% large cells for the SPC condition, compared to 49% large for 1G control cells. Therefore, taken together, a higher number of SPC-flown NSCs that with time acquired the large phenotype may be contributing to intracranial hypertension in astronauts during and after space flight. Data from the 3D-clinostat showed similar results but to a lesser extent where the net total increase was 27%.
The neural stem cell secretome produced in microgravity: is it beneficial or deleterious?
Models of cell replacement therapies for the CNS via NSCs grafting have been found beneficial not just because they may provide a missing or damaged cell type to the host, but in many cases, it is due to the molecules they secrete [15]. Therefore, the regenerative properties of the NSC secretome are being widely studied aiming at the identification of NSC-secreted factors to be used for neural regeneration and repair. All such studies have been performed on Earth in 1G. Here, we continue to build the knowledge on the effects of SPC-flown NSCs-secretome on naïve human embryo-derived NSCs. To our surprise, SPC-NSCs secretome, appears to be deleterious to naïve NSCs grown in 1G. Treatment with SPC-produced secretome resulted on enlargement of Earth-generated naïve NSCs, leading to hypertrophic cell body and in many cases death after 43 hours. In the same naïve-NSCs cultures the small cell population appeared to have survived such toxic effects. They formed chains of single cells but they either were devoid of cell processes or limited to a bipolar morphology, reminiscent of more primitive neural progenitors. The characterization of SPC-flown secretome, is currently being performed in our laboratory.
Cell memory and epigenetic changes
It has been reported that microgravity induces cellular and molecular “adaptations” including changes in the genome, the epigenome, and the proteome, among others [16]. One of six fundamental molecular and cellular features of space flight is epigenetics and gene regulation changes, many of which were discovered in the study of Scott Kelly’s yearlong stay onboard the ISS [14]. Yet, many of these changes reverted upon return to Earth [17]. Nonetheless, neither the intracranial hypertension nor the Neuro-ocular Syndrome revert upon astronauts’ return to Earth [6,7]. Our data derives from NSCs flown to space or NSCs cultured in sim-µG, however, they were studied after their exposure to microgravity. Therefore, our results indicate that microgravity exposed NSCs “remembered” having been in microgravity even though the study was performed in Earth gravity. Studies to ascertain which mechanism(s) are responsible for the changes reported here are of the essence. Nonetheless, we can hypothesize that changes in chromatin topography may be related to this phenomenon. Other studies using sorted cells or single cell experiments have shown differences in chromatin accessibility when comparing before and after space flight [18,19].
Conclusion
Neural stem cells are the focus of a large and growing field of research due to their unique and important capabilities involved with regeneration of neurons and glial cells. Here, we have investigated the impact of SPC-µG on NSCs and its differences with an alternate method of microgravity on Earth by using sim-µG produced by a 3D-clinostat. We have learned that NSCs display plasticity that allows them to adapt to weightlessness even when deprived of their niche and one of these changes is cell size. An effect is observed in both microgravity conditions that differ from the 1G control cells. Moreover, the effect of sim-µG on cell diameter shows the same tendency.
This study is meaningful to astronauts’ health because it proves for the first time that both types of microgravity, sim-µG and SPC-µG, increase the proportion of “large” NSCs. This subpopulation shift indicates that the changes caused by microgravity are permanent rather than transient because this study was performed after either space flight or clinostat exposure. Moreover, both types of microgravity increased the proliferation of NSCs compared to GC cells, supporting our hypothesis that microgravity generates more cells than Earth gravity.
While our resources for the present study did not allow us to ascertain in depth the possible cause for cell soma enlargement, it is known that normal morphology and physiology of cells depend in part on their cytoskeleton, whose components include actin, microtubules and intermediate filaments [20]. Cells under microgravity influence, display changes where the microtubules are shortened and curved. Less actin fibers but more condensed intermediate filaments are observed. It has been reported that mechanical unloading (i.e., microgravity exposure), affects normal human fibroblasts, resulting on changes in cell size, volume, morphology and adhesion features [21]. Similar changes have been reported for human macrophages [22]. Microgravity also influences bone-cell changes including a reduction of both transcription and translation of cytoskeleton and cytoskeleton-associated proteins, as well as decrease in focal adhesion which together lead to osteoclasts resorption pit formation [23,24].
Examples of alterations in response to microgravity such as viability, cell adhesion proliferation and migration in hematopoietic cells and thyroid cancer cells, have also been reported [25,26]. Simulated microgravity suppresses invasion and migration of human glioblastoma U87 cells [27]. Therefore, there is an array of possible responses to microgravity depending on the cell type and its condition i.e., normal or malignant that need to be characterized. Understanding the effects of microgravity on neural cells, particularly NSCs, will facilitate the design of measures to ensure astronauts’ health and safety on longer space missions and whilst at the International Space Station (ISS), and beyond.
Author’s Contribution
S.S., N.C., and V.T. performed extensive image analysis of time-lapse files, consolidated these data and helped to prepare some figure composites. C.C. helped with data analysis, interpretation, and manuscript writing. A.E-J. designed the entire study, chose the appropriate hardware, performed the work at Kennedy Space Center, recovered the cells from the space hardware upon their return to UCLA, performed image acquisition of live cells and thereafter global analysis of the data and prepared the manuscript.
Funding
We thank NASA Space Biology for Grant: NNX15AB43G; and The IDDRC Cell Culture Core is supported by NIH/ NICHD grant number U54HD087101-05.
Acknowledgments
We are extremely grateful to Mark Mobilia and the entire Zeiss team for their kind support with live imaging instruments with definite focus. We are equally thankful to Dr. Amy Rowat for support with the microscope system as live imaging and definite focus were a key component of our study. Dr. Fathi Karouia, Dr. Amy Gresser. Elizabeth Pane, and Medaya Torres for their help with the implementation of the study. The NASA Space Biology Project team at Ames Research Center Dr. D. Tomko, K. Sato, E. Taylor, ARC Space Biology Project Manager and the support personnel at the Space Station Processing Facility at Kennedy Space Center. Special thanks to Maria Birlem and Chriss Bruderrek from Yuri and STaARS team members Tom Kyler, BreAnne MacKenzie, Uli Kuebler from Airbus Defense and Space for introducing us to the specialized flying hardware. Dorwin Birt for continuous support with computer systems, Elida Escalante for preparation of the mesh carriers, and Aurora Espinosa de los Monteros for help preparing the final version of the manuscript. We thank the late Dr. Jean de Vellis for his enthusiasm and support of this novel research project.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
Declare conflicts of interest or state “The authors declare no conflict of interest.” Authors must identify and declare any personal circumstances or interest that may be perceived as inappropriately influencing the representation or interpretation of reported research results. Any role of the funders in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results must be declared in this section. If there is no role, please state “The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results”.
References
- Fernandez TS, Fernandez CS, Mencalha AL (2013) Human Induced Pluripotent Stem Cells from Basic Research to Potential Clinical Applications in Cancer Review Article. BioMed Research International 2013: 430290.
- Obernier K, Buylla AA (2019) Neural stem cells: origin, heterogeneity and regulation in the adult mammalian brain. Dev 146: 156059.
- Ma DK, Bonaguidi MA, Ming GL, Song H (2009) Adult neural stem cells in the mammalian central nervous system. Cell Res 19: 672-682.
- Zhang LF, Hargens AR (2018) Spaceflight-Induced Intracranial Hypertension and Visual Impairment: Pathophysiology and Countermeasures. Physiol Rev 98: 59-87.
- Ombergen AV, Demertzi A, Tomilovskaya E, Jeurissen B, Sijbers J, et al. (2017) The effect of spaceflight and microgravity on the human brain. J Neurol 264: 18-22.
- Lawley JS, Petersen LG, Howden EJ, Sarma S, Cornwell WK, et al. (2017) Effect of gravity and microgravity on intracranial pressure. J Physiol 595: 2115-2127.
- Lee AG, Mader TH, Gibson CR, Tarver W (2017) Space Flight-Associated Neuro-ocular Syndrome. JAMA Ophthalmol 135: 992-994.
- Studer M, Bradacs G, Hilliger A, Hürlimann E, Engeli S, et al. (2011) Parabolic maneuvers of the Swiss Air Force fighter jet F-5E as a research platform for cell culture experiments in microgravity. Acta Astronaut 68: 1729-1741.
- Shaka S, Carpo N, Tran V, Ma YY, Karouia F, et al. (2021) Human Neural Stem Cells in Space Proliferate more than GC Cells: Implications for Long-Term Space Travel. J Stem Cell Res Dev Ther 7: 069.
- Bradbury P, Wu H, Choi JU, Rowan AE, Zhang H, et al. (2020) Modeling the Impact of Microgravity at the Cellular Level: Implications for Human Disease. Front Cell Dev Biol 8: 96.
- Jeffrey AE, Nguyen K, Taniguchi A, Vergnes L, Vellis J (2016) Changes in energetics associated molecules, enhanced proliferation and oxygen metabolism in OLs grown in simulated microgravity, by the International Astronautical Congress (IAC). IAC-16,A1,7,6,X34299, Guadalajara, Mexico.
- Jeffrey AE, Paez PM, Cheli VT, Spreuer V, Wanner I, et al. (2013) Impact of Simulated Microgravity on Oligodendrocyte Development: Implications for Central Nervous System Repair. PLoS ONE 8: 76963.
- Espinosa A, Espejo D, Vellis JD (1997) Molecular Signaling and Regulation in Glial Cells. Springer Science and Business Media LLC, Berlin, Germany.
- Bakelman FEG, Darshi M, Green SJ, Gur RC, Lin L, et al. (2019) The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 364: 8650.
- Willis CM, Nicaise AM, Hamel R, Pappa V, Jametti LP, et al. (2020) Harnessing the Neural Stem Cell Secretome for Regenerative Neuroimmunology. Front Cell Neurosci 14: 590960.
- Demontis GC, Germani MM, Caiani EG, Barravecchia I, Passino C, et al. (2017) Human Pathophysiological Adaptations to the Space Environment. Front Physiol 8: 547.
- Afshinnekoo E, Scott RT, MacKay MJ, Pariset E, Cekanaviciute E, et al. (2020) Fundamental Biological Features of Spaceflight: Advancing the Field to Enable Deep-Space Exploration. Cell 183: 1162-1184.
- Gertz ML, Chin CR, Tomoiaga D, MacKay M, Chang C, et al. (2020) Multi-Omic, Single-Cell, and Biochemical Profiles of Astronauts Guide Pharmacological Strategies for Returning to Gravity. Cell Reports 2020: 26.
- Malkani S, Chin C, Cekanaviciute E, Mortreux M, Okunola H, et al. (2020) Circulating miRNA Spaceflight Signature Reveals Targets for Countermeasure Development. Cell Reports 33: 108448.
- Kopp S, Warnke E, Wehland M, Aleshcheva G, Magnusson NE, et al. (2015) Mechanisms of three-dimensional growth of thyroid cells during long-term simulated microgravity. Sci Rep 5: 16691.
- Buken C, Sahana J, Corydon TJ, Melnik D, Bauer J, et al. (2019) Morphological and Molecular Changes in Juvenile Normal Human Fibroblasts Exposed to Simulated Microgravity. Sci Rep 9: 11882.
- Thiel CS, Tauber S, Lauber B, Polzer J, Seebacher C, et al. (2019) Rapid Morphological and Cytoskeletal Response to Microgravity in Human Primary Macrophages. Int J Mol Sci 20: 2402.
- Nabavi N, Khandani A, Camirand A, Harrison RE (2011) Effects of microgravity on osteoclast bone resorption and osteoblast cytoskeletal organization and adhesion. Bone 49: 965-974.
- Bradbury P, Wu H, Choi JU, Rowan AE, Zhang H, et al. (2020) Modeling the Impact of Microgravity at the Cellular Level: Implications for Human Disease. Front Cell Dev Biol 8: 96.
- Grimm D, Koßmehl P, Shakibaei M, Tanzil GS, Pickenhahn H, et al. (2002) Effects of simulated microgravity on thyroid carcinoma cells. Journal of gravitational physiology: a journal of the International Society for Gravitational Physiology 9.: 253-256.
- Plett P, Abonour R, Reisner SF, Orschell C (2004) Impact of modeled microgravity on migration, differentiation, and cell cycle control of primitive human hematopoietic progenitor cells. Experimental hematology 32: 773-781.
- Shi ZX, Rao W, Wang H, Wang ND, Si JW, et al. (2015) Modeled microgravity suppressed invasion and migration of human glioblastoma U87 cells through downregulating store-operated calcium entry. Biochemical and biophysical research communications 457: 378-384.
Citation: Shaka S, Carpo N, Tran V, Cepeda C, Espinosa-Jeffrey A (2021) Microgravity Significantly Influences Neural Stem Cells Size and Numbers: Implications for Long-term Space Missions. J Stem Cell Res Dev Ther 7: 088.
Copyright: © 2021 Sophia Shaka, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

