
Molecular Characteristics of a Series of Clinical Isolates of Drug- Resistant Acinetobacter baumannii ST219 Strain: The Implications of a Sequence Analysis of the blaOXA-51-like
*Corresponding Author(s):
Satomi AsaiDepartment Of Laboratory Medicine, Tokai University School Of Medicine, Isehara, Kanagawa, Japan
Tel:+81-463-93-1121,
Email:sa@is.icc.u-tokai.ac.jp
Abstract
The drug-resistant Acinetobacter baumannii ST219 strain was sporadically isolated in the emergency intensive care unit of Tokai University Hospital in 2014 after an outbreak in 2013. The isolates were identical in their antimicrobial susceptibility pattern, the fingerprint pattern of rep-PCR, and the molecular properties including mutations in drug-resistant genes and decreased expression of the efflux pump and the outer membrane porin genes, but they were found to possess different sequence types based on the findings of multilocus sequence typing: ST208 and 219. The analysis of the blaOXA-51-likesequences showed that all were blaOXA-66. Given these findings, all of the isolates were considered to be subclones derived from the same strain. A sequence analysis of the blaOXA-51-like of A. baumannii would therefore be useful for investigating the relationship of nosocomial infections.
Keywords
INTRODUCTION
Acinetobacter baumannii is a strictly aerobic, non-fermenting, non-fastidious, non-motile, catalase-positive, oxidase-negative, gram-negative bacteria that is known to be a major pathogen of nosocomial infection in immunocompromised patients [1]. Outbreaks of antimicrobial-resistant A. baumannii have become a major clinical problem [1-3]. All strains of A. baumannii possessed a chromosomally encoded blaOXA-51-like, some of which provided resistance to carbapenems when the molecular milieu around the gene promoted its expression, such as ISAba1 [4]. The blaOXA-51-like has been reported to have sequence variations with over 40 variants [5,6].
We previously reported an outbreak of an amikacin- and ciprofloxacin-resistant A. baumannii (ST)219 strain that affected 15 patients in the emergency intensive care unit of Tokai University Hospital from September to October in 2013 [3]. Intensive control measures were implemented, including the replacement of the water supply system, which was considered to be a bacterial reservoir, and thus could successfully control the outbreak. However, sporadic cases of A. baumannii, with an identical pattern of antimicrobial susceptibility, were subsequently detected in 2014. The present study was undertaken to elucidate the molecular characteristics for antimicrobial resistance and typing for epidemiology in drug resistant (DR) A. baumannii-2014.
MATERIALS AND METHODS
Antimicrobial susceptibility testing, and screening of carbapenemase, MBL, ESBL and AmpC
Molecular typing
Multilocus Sequence Typing (MLST) was performed as described previously [9]. The MLST sequences were uploaded into the A. baumannii MLST sequence type database (http://pubmlst.org/abaumannii/) to determine the alleles and Sequence Types (ST). Clonal complexes (CCs) were assigned using the eBURST V3 software program (http://eburst.mlst.net/v3/) and defined as Single-Locus Variants (SLVs) and Double-Locus Variants (DLVs). The annealing temperature of the PCR amplification used in this study was 55ºC for gltA, gyrB, recA, and cpn60, and 50ºC for gdhB, gpi, and rpoD. The amplification products were purified with a QIAGEN DNA purification kit (QIAGEN GmbH). The DNA sequencing was performed using an ABI3500xL Genetic Analyzer (Applied Biosystems, Life Technologies Japan Ltd., Tokyo, Japan).
PCR assay for β-lactamase and armA
Sequencing of OXA-type β-lactamase and, gyrA and parC
Quantitative RT-PCR (qRT-PCR)
The data were analyzed using the StepOneTM software program. The expression of each target gene was normalized based on the level of the 16S rRNA mRNA gene and was expressed as a relative rate compared to that in the susceptible isolate of each pair. The expression of drug-susceptible A. baumannii (TS-A. baumannii-2014-7) was set as 1.0 [2]. The experiments were conducted at least three times independently, and all of the reactions were performed in triplicate.
RESULTS
Antimicrobial susceptibility testing, and screening of carbapenemase, MBL, ESBL and AmpC
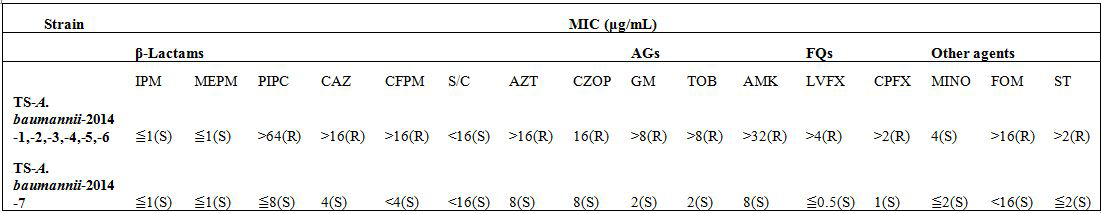
Molecular typing
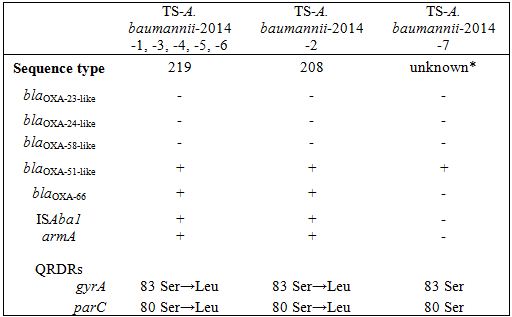
* TS-A. baumannii-2014-7 showed an unknown sequence type (gltA, gyrB, gdhB, recA, cpn60, gpi, rpoD: 15, 48, 58, 42, 36, 54, 41).
Characteristics of the regions involved in antimicrobial resistance
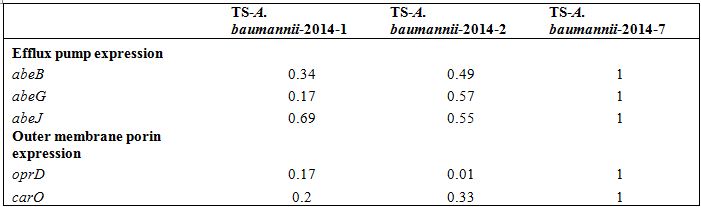 Table 3: Relative expression of efflux pumps and Outer membrane porin in clinical isolates Acinetobacter baumannii by quantitative RT-PCR.
Table 3: Relative expression of efflux pumps and Outer membrane porin in clinical isolates Acinetobacter baumannii by quantitative RT-PCR.DISCUSSION
Clinical isolates of an outbreak in 2013 and subsequent sporadic detection in 2014 of DR-A. baumannii in the emergency intensive care unit of Tokai University Hospital showed resistance to a broad spectrum of antimicrobials except for carbapenems, and were found to have blaOXA-51-like carrying blaOXA-66 with ISAba1, alterations of genes responsible for AGs and FQs, and decreased expression of the efflux pump and outer membrane porin-encoding genes. These findings suggested that these isolates of A. baumanniishared a molecular basis for the same susceptibility pattern to antimicrobials. These findings are compatible with collateral susceptibility to carbapenem and consistent with a previous report indicating that the combination of OXA-type β lactamases with ISAba1 and deficiency of outer membrane porins deficiency alone does not confer carbapenem resistance, and that overexpression of the efflux pumps may be necessary [2,12,22].
Isolates of TS-A. baumannii-2014-1 to -6 showed identical molecular characteristics, such as the fingerprint pattern, OXA type, drug resistant genes and expression of efflux pumps. A. baumannii can survive for long-term periods of time in the hospital environment, causing sporadic and endemic infection [2,3,23]. We experienced an outbreak twice in the past in the emergency intensive care unit and burn unit of our University Hospital. DR-A. baumannii ST208 was involved in an outbreak in 2011, where the air fluidity bed was identified as a reservoir. An outbreak of DR-A. baumannii in 2013 was detected from the water systems including hands-free automatic tap and water mixture side of the joint tube. Effective measures to minimize the risk in the wet environmental reservoir included strict sanitary management of the water systems in order to prevent future outbreaks. The MLST sequences between ST208 and ST219 differed by only a single gpi base, and these isolates closely resembled one another in the molecular characteristics for resistance against each drug. This finding suggested that the ST208 and ST219 isolates were closely related in terms of genetics and might have been derived as subclones from the same origin.
In the detection of subsequent isolates, a reservoir was not identified despite environmental sampling for bacterial culture at several times. A more intensive environmental surveillance will be needed to identify the reservoir should DR-A. baumannii-2014 continue to be detected. However, the reinforcement of environmental disinfection, including clinical surfaces, and ensuring hand hygiene with alcohol containing antiseptic will be crucial for reducing the risk of cross-transmission in healthcare facilities [24].
In conclusion, a detailed molecular analysis of DR-A. baumannii would provide important knowledge for controlling nosocomial infections. Even when a different ST strain is detected, the sequencing of the blaOXA-51-like would be useful for determining the clonality, which would thus make it possible to identify the relationship of A. baumannii infection.
ACKNOWLEDGMENTS
This work was supported by JSPS KAKENHI Grant Numbers 25670768 and 26460657.
REFERENCES
- Dijkshoorn L, Nemec A, Seifert H (2007) An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol 5: 939-951.
- Asai S, Umezawa K, Iwashita H, Ohshima T, Ohashi M, et al. (2014) An outbreak of blaOXA-51-like- and blaOXA-66-positive Acinetobacter baumannii ST208 in the emergency intensive care unit. J Med Microbiol 63: 1517-1523.
- Umezawa K, Asai S, Ohshima T, Iwashita H, Ohashi M, et al. (2015) Outbreak of drug-resistant Acinetobacter baumannii ST219 caused by oral care using tap water from contaminated hand hygiene sinks as a reservoir. Am J Infect Control 43: 1249-1251.
- Evans BA, Amyes SG (2014) OXA β-lactamases. Clin Microbiol Rev 27: 241-263.
- Poirel L, Naas T, Nordmann P (2010) Diversity, epidemiology, and genetics of class D beta-lactamases. Antimicrob Agents Chemother 54: 24-38.
- Zander E, Nemec A, Seifert H, Higginsa PG (2012) Association between β-lactamase-encoding blaOXA-51 variants and DiversiLab rep-PCR-based typing of Acinetobacter baumannii isolates. J Clin Microbiol 50: 1900-1904.
- Clinical and Laboratory Standards Institute (2010) Performance standards for antimicrobial susceptibility testing, 20th Informational supplement, CLSI, Wayne, PA.
- Mohapatra BR, Broersma K, Mazumder A (2007) Comparison of five rep-PCR genomic fingerprinting methods for differentiation of fecal Escherichia coli from humans, poultry and wild birds. FEMS Microbiol Lett 277: 98-106.
- Fu Y, Zhou J, Zhou H, Yang Q, Wei Z, et al. (2010) Wide dissemination of OXA-23-producing carbapenem-resistant Acinetobacter baumannii clonal complex 22 in multiple cities of China. J Antimicrob Chemother 65: 644-650.
- Woodford N, Ellington MJ, Coelho JM, Turton JF, Ward ME, et al. (2006) Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents 27: 351-353.
- Turton JF, Ward ME, Woodford N, Kaufmann ME, Pike R, et al. (2006) The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol Lett 258: 72-77.
- Lee Y, Yum JH, Kim CK, Yong D, Jeon EH, et al. (2010) Role of OXA-23 and adeABC efflux pump for acquiring carbapenem resistance in an Acinetobacter baumannii strain carrying the blaOXA-66 gene. Ann Clin Lab Sci 40: 43-48.
- Yamane K, Wachino J, Doi Y, Kurokawa H, Arakawa Y (2005) Global spread of multiple aminoglycoside resistance genes. Emerg Infect Dis 11: 951-953.
- Endo S, Yano H, Hirakata Y, Arai K, Kanamori H, et al. (2012) Molecular epidemiology of carbapenem-non-susceptible Acinetobacter baumannii in Japan. J Antimicrob Chemother 67: 1623-1626.
- Liu YH, Kuo SC, Lee YT, Chang, IC, Yang SP, et al. (2012) Amino acid substitutions of quinolone resistance determining regions in gyrA and parC associated with quinolone resistance in Acinetobacter baumannii and Acinetobacter genomic species 13TU. J Microbiol Immunol Infect 45: 108-112.
- Zander E, Chmielarczyk A, Heczko P, Seifert H, Higgins PG (2013) Conversion of OXA-66 into OXA-82 in clinical Acinetobacter baumannii isolates and association with altered carbapenem susceptibility. J Antimicrob Chemother 68: 308-311.
- Fernando D, Kumar A (2012) Growth phase-dependent expression of RND efflux pump- and outer membrane porin-encoding genes in Acinetobacter baumannii ATCC 19606. J Antimicrob Chemother 67: 569-572.
- Peleg AY, Seifert H, Paterson DL (2008) Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21: 538-582.
- Hou PF, Chen XY, Yan GF, Wang YP, Ying CM (2012) Study of the correlation of imipenem resistance with efflux pumps adeABC, AdeIJK, AdeDE and AbeM in clinical isolates of Acinetobacter baumannii. Chemotherapy 58: 152-158.
- Srinivasan VB, Rajamohan G, Pancholi P, Marcon M, Gebreyes WA (2011) Molecular cloning and functional characterization of two novel membrane fusion proteins in conferring antimicrobial resistance in Acinetobacter baumannii. J Antimicrob Chemother 66: 499-504.
- Coyne S, Rosenfeld N, Lambert T, Courvalin P, Périchon B (2010) Overexpression of resistance-nodulation-cell division pump adeFGH confers multidrug resistance in Acinetobacter baumannii. Antimicrob Agents Chemother 54: 4389-4393.
- Rumbo C, Gato E, López M, Ruiz de Alegría C, Fernández-Cuenca F, et al. (2013) Contribution of efflux pumps, porins, and β-lactamases to multidrug resistance in clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother 57: 5247-5257.
- Dijkshoorn L, Nemec A, Seifert H (2007) An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol 5: 939-951.
- WHO (2016) Clean Care is Safer Care. First Global Patient Safety Challenge, World Health Organization, Geneva, Switzerland.
Citation: Umezawa K, Asai S, Iwashita H, Minakawa Y, Ohashi M, et al. (2016) Molecular Characteristics of a Series of Clinical Isolates of Drug-Resistant Acinetobacter baumannii ST219 Strain: The Implications of a Sequence Analysis of the blaOXA-51-like. J Emerg Med Trauma Surg Care 3: 017.
Copyright: © 2016 Kazuo Umezawa, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

