Not Out of the Woods yet-a Case of Recurrent Euglycemic Diabetic Ketoacidosis Secondary to Empagliflozin and Pancreatitis
*Corresponding Author(s):
Marvin Wei Jie ChuaDepartment Of General Medicine, Consultant Endocrinologist, Sengkang General Hospital, 110 Sengkang East Way, Singapore 544886, Singapore
Tel:+65 69302346,
Email:marvin.chua.w.j@singhealth.com.sg
Abstract
Since their introduction, Sodium-Glucose Co-Transporter-2 (SGLT2) inhibitors have demonstrated a multitude of exceptional clinical benefits. Indeed, they have transcended their initial pharmacological category of anti-diabetic medications following landmark clinical trials which demonstrated their benefits on cardiovascular outcomes, renal outcomes and all-cause mortality, both in patients with and without Diabetes [1-4]. It is almost certain that we will see increasingly widespread prescription of SGLT2 inhibitors.
In the midst of this excitement, the clinician should keep in mind a rare but potentially life-threatening complication of SGLT2 inhibitors-euglycemic diabetic ketoacidosis. I describe a patient with likely pancreatogenic diabetes mellitus who was started on empagliflozin and admitted one month later with euglycemic diabetic ketoacidosis and acute pancreatitis. Following initial resolution, the patient developed an unexpected recurrence of ketoacidosis which was likely related to prolonged effect of the SGLT2 inhibitor and recurrent pancreatic inflammation-hence the title of this report.
Keywords
Empagliflozin; Euglycemic diabetic ketoacidosis; Pancreatitis; SGLT2 inhibitors
Introduction
While Sodium Glucose Co-Transporter 2 (SGLT2) inhibitors have demonstrated impressive clinical benefits, we should maintain vigilant when prescribing. I report a patient with euglycemic diabetic ketoacidosis (eDKA) precipitated by empagliflozin and pancreatitis, which recurred following initial resolution. This case demonstrates several points of practical relevance to clinicians.
Case Presentation
The patient is a 43 year old male with Diabetes Mellitus diagnosed at age 36, for which he was initially treated with glipizide. The patient developed an episode of acute necrotizing pancreatitis in 2015, for which investigations revealed Common Bile Duct (CBD) dilatation with no calculi. Due to worsening glycaemic control following this episode of pancreatitis, he was subsequently switched to twice-daily biphasic insulin aspart (Novomix) with metformin in June 2015, and was on follow up with his General Practitioner (GP). Although HbA1c and glucose readings were not available, the patient’s GP had informed him that his glycaemic control was suboptimal, prompting the addition of empagliflozin 10 mg daily to his regime in December 2020. He had no known cardiac or renal disease, retinopathy or neuropathy.
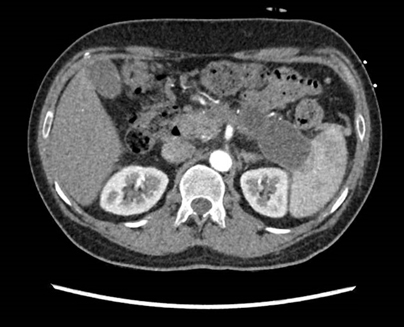
One month later, the patient was admitted to our institution with epigastric pain radiating to the back and vomiting of 2 days duration. There was no fever, diarrhea or other symptoms of infection, and the patient had abstained from alcohol. On initial assessment, he was afebrile (temperature 36.3 degrees Celsius), blood pressure was 153/90 mm Hg, heart rate was 91 beats per minute and oxygen saturation was 98% on room air. Clinical examination revealed epigastric tenderness with no rebound or guarding. Examination of the cardiovascular, respiratory and neurological systems was unremarkable. Biochemical investigations showed elevated amylase and lipase with ketonemia, high anion gap metabolic acidosis and normoglycemia, while CT of the abdomen revealed peripancreatic inflammatory stranding with a 5 mm pancreatic duct calculus (Table 1). The clinical presentation and investigations were consistent with eDKA and acute pancreatitis secondary to pancreatic duct calculus. As the patient was haemodynamically stable with no local complications, systemic complications or organ failure, the modified Marshall score was 0, consistent with mild acute interstitial edematous pancreatitis.
|
Investigations |
Results |
Reference Range |
Remarks |
|
Serum Glucose (mmol/L) |
10.5 |
3.9 -11.0 |
Normal |
|
Beta-hydroxybutyrate (mmol/L) |
5.4 |
0.0 - 0.6 |
Elevated |
|
Blood gas and biochemistry profile |
|||
|
pH |
7.28 |
7.35- 7.45 |
Decreased |
|
Bicarbonate (mmol/L) |
14.4 |
21.0 - 27.0 |
Decreased |
|
pC02 (mm Hg) |
26.0 |
35.0 - 45.0 |
Decreased |
|
Base Excess (mmol/L) |
-12.9 |
- 2.0 - 2.0 |
Decreased |
|
Sodium (mmol/L) |
131 |
136- 146 |
Decreased |
|
Chloride (mmol/L) |
95 |
98 - 107 |
Decreased |
|
Anion Gap |
21.6 |
8 - 12 |
Elevated |
|
Potassium (mmol/L) |
5.0 |
3.5 - 5.1 |
Normal |
|
Urea(mmol/L) |
6.5 |
2.7 - 6.9 |
Normal |
|
Creatinine (umol/L) |
71 |
59 - 104 |
Normal |
|
Pancreatic enzymes |
|||
|
Amylase (U/L) |
318 |
38 - 149 |
Elevated |
|
Lipase (U/L) |
692 |
13 - 60 |
Elevated |
|
CT abdomen and pelvis |
|||
|
Peripancreatic inflammatory stranding is seen. The pancreas demonstrates some degree of atrophy. The main pancreatic duct is prominent and a 5 mm calculus is noted at the pancreatic duct insertion. Pseudocysts of uncertain chronicity are seen adjacent to the distal body and tail of pancreas. Slight prominence of central intrahepatic ducts is seen. There are no gallbladders or biliary ductal calculi. No suspicious hepatic lesion is detected. The spleen appears unremarkable. |
|||
|
Liver function |
|||
|
Albumin (g/L) |
47 |
40 - 51 |
Normal |
|
Total bilirubin (umol/L) |
12 |
7 - 32 |
Normal |
|
Alanine transaminase (U/L) |
15 |
6 -66 |
Normal |
|
Aspartate transaminase (U/L) |
31 |
12 - 42 |
Normal |
|
Alkaline phosphatase (U/L) |
77 |
39 - 99 |
Normal |
|
Other investigations |
|||
|
HbA1c (%) |
8.2 |
4.6 - 6.4 |
Elevated |
|
Triglycerides (mmol/L) |
0.94 |
<1.7 - 2.2 |
Normal |
|
Corrected calcium (mmol/L) |
2.18 |
2.09 - 2.46 |
Normal |
|
Endoscopic Retrograde Cholangiopancreatography |
|||
|
Cholangiogram shows no CBD stone. Pancreatogram shows no stone with multiple strictures along the pancreatic duct. Stents inserted into pancreatic duct and CBD. |
|||
Table 1: Investigations for diagnosis of euglycemic diabetic ketoacidosis and acute pancreatitis, as well as related investigations.
The patient was started on intravenous insulin infusion and fluids with close monitoring of serum electrolytes. Empagliflozin was stopped. Following close to 2 days of IV insulin infusion, acidosis had resolved but there was persistent ketonemia. This was attributed to nil oral in take, and the patient was switched to subcutaneous insulin and allowed diet. However, severe abdominal pain occurred the following day. Investigations confirmed the recurrence of ketoacidosis for which IV insulin infusion was resumed and IV Dextrose 10% fluid was started (Table 2).The patient remained haemodynamically stable. The increase in risk of procedure-related pancreatitis notwithstanding, the decision was made to proceed with Endoscopic Retrograde Cholangiopancreatography (ERCP), which was performed uneventfully. No calculi were found on ERCP despite initial CT findings of a pancreatic duct calculus, which could be explained by spontaneous passage of the calculus (Table 2). Subsequently, the patient remained well with no ketonemia or acidosis and was converted to subcutaneous insulin. empagliflozin continued to be held off on discharge.
|
Time (hours) |
Capillary Blood Glucose (mmol/L) |
IV Insulin Infusion (units/hour) |
IV Fluid |
pH |
HC03 (mmol/L) |
Beta-hydroxybutyrate (mmol/L) |
Remarks |
|
Day 1 |
|||||||
|
1400 - 1700 |
10.2 - 12.2 |
2 |
Dextrose 5% Sodium Chloride (0.9%) 125 ml/hour |
7.28 |
14.4
|
4.2 - 5.4 |
IV insulin infusion started |
|
1700 - 0000 |
8.3 - 10.0 |
1 |
7.34 |
17.7 |
2.3 |
|
|
|
Day 2 |
|||||||
|
0000- 0400 |
7.5 -9.7 |
1 |
Dextrose 5% Sodium Chloride (0.9%) 125 ml/hour |
7.34 |
19.2 |
1.2 |
|
|
0400 - 1700 |
5.9 - 7.3 |
0.5 |
Dextrose 5% Sodium Chloride (0.9%) 83.3 ml/hour |
7.34 -7.36 |
19.4 - 20.0 |
1.2- 1.3 |
|
|
1700 -2300 |
7.8 - 10.5 |
1 |
7.37 |
18.4 |
3.0 |
|
|
|
2300- 0000 |
8.3 |
0.5 |
Lactated Ringer’s solution 41.7 ml/hour |
N/A |
1.4 |
|
|
|
Day 3 |
|||||||
|
0000 - 0300 |
4.7- 5.7 |
0.5 |
Lactated Ringer’s solution 41.7 ml/hour |
7.39 |
21.6 |
1.3 |
IV insulin infusion stopped and switched to subcutaneous insulin. Allowed diet |
|
Day 4 |
|||||||
|
2000- 2200 |
9.4 - 11.9 |
2 |
Dextrose 5% Sodium Chloride (0.9%) 83.3 ml/hour |
7.33 |
17.6 |
4.6 |
Recurrence of abdominal pain. Restarted onIV insulin infusion |
|
2200 - 0000 |
8 - 8.6 |
1 |
N/A |
N/A |
|
||
|
Day 5 |
|||||||
|
0000- 1100 |
5.8 - 7.9 |
0.5 |
Dextrose 5% Sodium Chloride (0.9%) 83.3 ml/hour |
7.34 - 7.36 |
18.9-20.4 |
2.9 - 4.1 |
|
|
1100- 0000 |
7.1 - 10.1 |
1 |
Dextrose 10% 83.3 ml/hour |
7.36 |
20.2- 22.7 |
0.4 - 2.7 |
Switched to IV Dextrose 10% drip |
|
Day 6 |
|||||||
|
0000 -1700 |
9.1 - 10.3 |
1 |
Dextrose 10% 83.3 ml/hour |
7.36 |
22.6 |
0.2 - 0.4 |
ERCP done at 1600 hrs |
|
1700 -1900 |
8.9 - 10.4 |
1 |
7.39 |
22.7 |
|
||
|
1900 -0000 |
10 - 11.6 |
2 |
N/A |
N/A |
|
||
|
Day 7 |
|||||||
|
0000 - 1430 |
9.4 - 12.0 |
1 |
Dextrose 10% 83.3 ml/hour |
N/A |
N/A |
0.2 |
IV insulin infusion and dextrose drip stopped. Allowed diet |
Table 2: Trend of capillary glucose, ketones, pH and HC03 with corresponding IV fluid and IV insulin infusion rate.
Discussion
SGLT2 inhibitors and acute pancreatitis have both been associated with eDKA [5,6]. To the best of my knowledge, this is the first reported case of eDKA simultaneously due to empagliflozin and acute pancreatitis. SGLT2 inhibitors lead to increased glycosuria and reduction of the insulin-to-glucagon ratio, which stimulates lipolysis and ketogenesis [5,6]. With worsening insulin deficiency or restriction of carbohydrate intake, ketosis may deteriorate into ketoacidosis [5], both factors, particularly the latter, were relevant in this patient. Although resuming oral intake initially led to resolution of persistent ketosis, the ensuing pancreatic exocrine activity likely led to recurrent pancreatic inflammation and ketoacidosis despite ongoing therapy with subcutaneous insulin. This was supported by the observed resolution of recurrent ketoacidosis following the switch to parenteral carbohydrate administration.
There is currently no consensus regarding the optimal timing of resumption of oral feeding in acute pancreatitis. While enteral nutrition is believed to stimulate release of pancreatic exocrine enzymes and possibly prolong recovery, it might also help maintain the gut protective barrier and reduce bacterial translocation and sepsis [7,8], In patients with eDKA secondary to both SGLT2 inhibitor and acute pancreatitis, due to the possible risk of recurrent pancreatic inflammation and ketoacidosis, the clinician might wish to opt for a more judicious approach to resuming oral feeding.
Another important reason for recurrent ketoacidosis is the prolonged effect of SGLT2 inhibitors, which have been described to lead to prolonged glycosuria and ketonemia [9,10]. Suggested mechanisms include active drug secretion into the renal proximal tubule, drug binding to plasma proteins in an acidic environment and dissociation following resolution of acidosis, and receptor down-regulation [11-13]. Thus, prolonged treatment and increased vigilance for recurrence following resolution of SGLT2 inhibitor-associated eDKA may be required.
Should empagliflozin have been started? As with Type 1 Diabetes but perhaps less well-known, SGLT2 inhibitors should be prescribed with caution in patients with pancreatic disease including previous pancreatitis [5,14,15]. Prior to initiation, a detailed risk-benefit analysis and explanation of the necessary precautions should be undertaken beforehand.
Conclusion
In conclusion, this case highlights the following clinical pearls in the management of eDKA secondary to SGLT2 inhibitor and acute pancreatitis:
- eDKA secondary to SGLT2 inhibitor and acute pancreatitis is an uncommon but life-threatening entity which requires prompt recognition and treatment.
- In addition to the usual management principles for DKA, an important component of management is adequate provision of carbohydrates. In this specific clinical scenario, the benefits of oral feeding should be balanced with the possible risk of recurrent pancreatic inflammation and ketoacidosis.
- Prolonged therapy might be required, and the clinician should remain vigilant for recurrence of DKA following initial resolution.
- Prior to initiation of SGLT2 inhibitors, clinicians should carefully review the patient’s medical history for any contraindications, including a previous history of pancreatic disease or pancreatitis.
Declaration
- • The author declares that there is no funding source.
- • The author declares that there is no conflict of interest.
- • The author declares that there is no acknowledgement.
- • Informed consent was obtained from the patient for publication of this manuscript.
References
- Edwards JL (2016) Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 374: 1093.
- Wiviott SD, Raz I, Bonaca MC, Mosenzen O, kato ET, et al. (2019) Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 380: 347-357.
- Neal B, Perkovic V, Matthews DR (2017) Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 377: 2097-2099.
- McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, et al. (2019) Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 381: 1995-2008.
- Rosenstock J, Ferrannini E (2015) Euglycemic Diabetic Ketoacidosis: A Predictable, Detectable, and Preventable Safety Concern With SGLT2 Inhibitors. Diabetes Care 381: 63-1642.
- Bonora BM, Avogaro A, Fadini GP (2020) Euglycemic Ketoacidosis. Curr Diab Rep 20: 25.
- Vaughn VM, Shuster D, Rogers MAM, Mann J, Conte ML, et al. (2017) Early Versus Delayed Feeding in Patients With Acute Pancreatitis: A Systematic Review. Ann Intern Med 166: 883-892.
- Zhang J, Zhu S, Tan D, Ma A, Yang Y, et al. (2019) A meta-analysis of early oral refeeding and quickly increased diet for patients with mild acute pancreatitis. Saudi J Gastroenterol 25: 14-19.
- Aggarwal A, Jainn A, Sachdeva S, Kulairi ZI (2020) Prolonged Glucosuria With Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors: A Case Report and Review of Literature. Cureus 12: e11554.
- Pujara S, Ioachimescu A (2017) Prolonged Ketosis in a Patient With Euglycemic Diabetic Ketoacidosis Secondary to Dapagliflozin. J Investig Med high Impact Case Rep 5.
- Liu JJ, Lee T, DeFronzo RA (2012) Why Do SGLT2 inhibitors inhibit only 30-50% of renal glucose reabsorption in humans. Diabetes 61: 2199-2204.
- Devineni D, Vaccaro N, Polidori D, Rusch S, Wajs E, (2014) Effects of hydrochlorothiazide on the pharmacokinetics, pharmacodynamics, and tolerability of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in healthy participants. Clin Ther 36: 698-710.
- Hinderling PH, Hartmann D (2005) The pH dependency of the binding of drugs to plasma proteins in man. Ther Drug Monit 27: 71-85.
- Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, et al. (2017) American association of clinical endocrinologists and american college of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract 23: 1-87.
- Zhi M, Zhu X, Lugea A, Waldron RT, Pandol SJ, et al. (2019) Incidence of New Onset Diabetes Mellitus Secondary to Acute Pancreatitis: A Systematic Review and Meta-Analysis. Front Physiol 10: 637.
Citation: MWJ Chua (2021) Not Out of the Woods yet-a Case of Recurrent Euglycemic Diabetic Ketoacidosis Secondary to Empagliflozin and Pancreatitis. J Diabetes Metab Disord 8: 038.
Copyright: © 2021 Marvin Wei Jie Chua, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.