
Open Abdomen and Temporarily Abdominal Closure
*Corresponding Author(s):
Abdelkader BoukerroucheDepartment Of Digestive Surgery, Hospital Of Beni-Messous, University Of Algiers, Algiers, Algeria
Tel:+213 661 22 72 98,
Email:aboukerrouche@yahoo.com
Abstract
Aggressive resuscitation combined with staged surgery in severely injured patients has increased the incidence of abdominal compartment syndrome (ACS) leading to the development ofopen abdomen strategy (OA).The open abdomen (OA) is a planned management strategy implemented in critically injured patientsthatneed re-laparotomy to complete definitive surgery. Damage control surgery associated with damage control resuscitation and open abdomen resulted in eradicating the postoperative ACS in critically injured patients.
Additionally, OA implementation has been expanded to non-traumatic abdominal conditions. However, OA is a morbid condition with associated complications including principally intestinal fistula, abdominal fascia retraction, visceral adherences and ventral hernia. Temporarily abdomen closure is planned strategy for OA management aiming to minimize OA associated complications and increase abdominal fascia closure rate. Multiple temporarily abdomen closure techniques have been described, and vacuum assisted closure techniqueis commonly the most used technique with high rate of fascial closure and lower rate of complications.
Keywords
Abdominal fascia closure;Damage control surgery;Damage control resuscitation; Open abdomen
ABBREVIATIONS
ACS:Abdominal Compartment Syndrome; DCR: Damage Control Resuscitation; DCS:Damage Control Surgery; ECF: Enterocutaneous Fistula; FCT: Fascial Closure Technique; GCS: Glasgow Coma Score; IAH:Intra-Abdominal Hypertension; IAP: Intra-Abdominla Pressure; ICU: Intensive Care Unit; ISS: Injury Severity Score; NMBA: Neuromuscular Blockade Agent; OA:Open Abdomen; PRBC: Packed Red Blood Cells; PTFE: Polytetrafluoroethylene; TAC: Temporarily Abdominal Closure; WP: Wittmann Patch
INTRODUCTION
Performing definitive surgery in severely injured patients was reputed to bedetrimentaltooutcomes [1]. So, delaying reconstruction and performing staged surgery with continuous resuscitation resulted in increasing patient survival with physiology derangement [2].Despite the survival improvement, staged surgeryor damage control surgeryassociated with abdominal fascial closureand large volume resuscitation hasincreasedtheincidenceof abdominal compartment syndrome (ACS) leading to increasedmortalityin survived patients [3]. Very large fluid resuscitation and primary abdominal wall closure following damage control surgery have been recognized as predictor factors for ACS development [4,5]. Also, damage control resuscitationtechnique (DCR), based on fluid restrictionand massive transfusion of blood products, has increasinglycontributedto reducingACS incidence [6-8].
The open abdomen (OA)strategyimplementedwith DCR followingdamage control surgeryorin high- risk patientofintra-abdominalhypertension (IAH) developmenthas led to almost eradication ofACS in critically injured patients[6,7,8].Therefore, DCS, DCR and OAform the moderncare trauma in severely injured patients.In addition, DCSand OAapproachesare being implemented in non-trauma condition includingsevere abdominalsepsis, repeated abdominal surgeryand secondary ACS.The increasing prevalence of OA and associated morbidity has prompted to develop multiple techniques of temporarily abdominal closure (TAC).However, the ideal TAC methodshould contain the abdominal content, prevent abdominal cavity contamination, visceradesiccation, evisceration and visceral adherences, facilitate peritonealfluidevacuation, allow easy access to the abdominal cavity,and prevent abdominal fascia retraction and recurrent ACS [9-11].Resuscitation of patient with OA should be continued including restoration of normal physiology with judicious fluid management and introduction of early enteral nutrition support. So, the optimal goal of early OA management is to facilitate early closure (within one week) and prevent associated complications including failure of the primary fascial closure, intestinal fistula, abdominal sepsis and ventral hernia.
DEFINITION OF OPEN ABDOMEN
The open abdomen (OA) is defined as an abdomen with unapproximated fascial edges to prevent intra-abdominal hypertension (IAH) or abdominal compartment syndrome in patients who are subjected to repeat operation. The OA strategy is implemented in severe abdominal trauma following damage control surgery, severe abdominal sepsis, abdominal wall necrosis, abdominal compartment syndrome and acute mesenteric ischemia [12].
The OA leads to abdominal wall retraction with high risk of abdominal cavity infection, visceral adherences and intestinal fistula formation with the need for further abdominal wall reconstruction. Theses multiple conveniences have led to the development of the temporarily abdominal closure concept.Temporarily abdominal closure (TCA) is a planned surgical management strategy implemented in patients with OA. So, the abdominal wall is temporarily closed by using several methods (skin or artificial materials) in order toprotect the abdominal organs, avoid peritoneal cavity infection and reduce abdominal fascia and skin retraction [12].
INDICATION AND RISK FACTORS OF OA
Early decision to perform damage control surgery (DCS) may decrease mortality [13].So, surgical trauma team should rapidly make decision whether performing definitive surgery or staged surgery, sometimes upon entering the operating room. Damage control surgery approach includes bleeding control, contained abdominal contamination, and delayed reconstructive surgery allowingresuscitation to optimize patient conditions.When implementing DCS, abdominal fascia closure is often delayed resulting in open abdomen because of the need to perform repeated laparotomy. So, iterative laparotomy associated with abdominal wall closureincreases the risk of abdominal compartment syndrome (ACS).
Open abdomen strategy can be applied in patients with severe trauma injury and traumatic shock who need repeated celiotomy to perform complete surgery after damage control surgery.Also, OA can be indicated insevere abdominal sepsis following pancreatic necrosis, gastrointestinal tract injury or fistula with planned relaparotomy[14,15].Severe abdominal wall injury,intra-abdominal hypertension (IAH) or ACSwith needfor decompressive laparotomy, and patients at high risk for postoperative ACS,are also an indication for implementingOA approach [16-18].
While managing severe trauma with hemorrhagic shock, damage control surgery approach is often implemented in association with OA strategy because DCS and OA strategy have almost the same risk factors. These common risk factors includeintraoperative predictors such as exsanguination requiring transfusion of 10 units of packed red blood cells (PRBC) [19], physiologic derangement as ascertained by biologic values, acidosis (pH\7.2 ), hypothermia (temperature\34 C), and coagulopthy (prothrombin time: 16) [20,21].
Also, prolonged operative procedure (> 90 min), difficulties to make the appropriate decision for treatment and limited technical conditions are the risk factors for applying DCS and OA strategy.Preoperative predictors of DCSalso include high severity injury score (ISS ≥25) associated with severe hemorrhagic shock (diastolic pressure <70 mmHg), low Glasgow Coma Score (GCS), and hypothermia, and poor clotting function [21].Therefore,any of these factors should prompt the surgeon to perform a staged surgerywithOA or TACin severely trauma patient undergoingopen laparotomy.However, the surgical management strategy must be based on the dynamic response to resuscitation, and damage control strategy remains primordial in the management of shocked patients or those not responding to intensive resuscitation[22].
TEMPORARY ABDOMINAL CLOSURE OPTIONS
Initially, skin or fascial closure was employed to enhance the packing effect used to control bleeding particularly in liver trauma; however, this resulted in increasing the ACS incidence leading to the limited use of this treatment method [4,23]. The increase in ACS incidence following damage control surgery with abdominal wall closure has led to the development of the open abdomen strategy. The temporarily abdominal closure aims to minimize the OA consequences.In addition, the surgical techniques have been increasingly refined focusing on stabilizing the abdominal compartment and allowing swift, convenient, removal and replacement of the temporary system.So, the ideal TAC would maintainabdominal viscera in homeostatic environment, limit peritoneal contamination, prevent bowel trauma, adherence formation and recurrent ACS, andalso minimize skin and fascia trauma .Importantly,the TACwould prevent abdominal wall retractionand facilitate further primary fascial closure [9].Indeed, the use of any temporary closure device should protect the underlying bowel and allowingeasy access to the peritoneal cavity.Numerous TAC devices both commercial and self-designed are available for surgeons. Skin closure techniques, fascial closure techniques and negative pressure therapy are the available options to achieve TAC. There is no evidence-based preferable technique; however, the vacuum assisted closure technique is the most commonly used technique with high rate of abdominal fascial closure and lower complication rate. In addition, the combined use of fascial closure technique and vacuum assisted techniques wasassociated with a very high primary fascia closure (>86%)
Skin closure techniques
The skin closure techniques had their place in the early days of the TAC, including simple running suture of the skin, sequential towel clip closure, the silo technique, and the Bogota bag. Tower clip and suture closure of the skin are rapid and inexpensive; however, they are associated with increased risk of evisceration, skin necrosis, infection, and recurrent ACS (13% to 36%) thathave ledtotheir no use nowadays[24].The silo technique and Bogota bagconsists in suturing an inert non permeable barrier (sterile IV bag,bowel bag, Steri-Drape, Silastic cloth) to the skin or fascia in order to contain abdominal viscera. These inexpensive techniques with swift application allow some abdominal stabilization. However both techniques are prone to leakage, visceral adherences, evisceration and do not prevent abdominal fascial retraction [9,24].The primary abdominal wall closure rates vary from 12 to 82% with ACS incidence ranging from 2.3 to 33.0% [25]. The enterocutaneous fistula (ECF) ratesare lowervaryingfrom 0 to 14.4%.
Fascial closure techniques
The fascial closure technique (FCTs) is to suture grafting materialsto the abdominal wall fascia, aiming to protect the abdominal viscera and allow progressive fascial approximation and closure [26,27]. Materials used include nonabsorbable meshes such as polypropylene mesh (Marlex mesh), Wittmann mesh, expanded polytetrafluoroethylene (ePTFE) mesh, polypropylene and ePTFE composite mesh, and absorbable meshes such as Vicryl or biological mesh.
When the non-absorbable mesh is used,the greater omentum should be placedto cover the bowel if at all possible in order to avoid direct contactbetweennon absorbable material andbowel. The graft material should be redundant to prevent ACS development; it is gradually tightened by excising and suturing the central portion of the graft to facilitate fascial approximation in the postoperative stage [28-32]. Typically, the tightening is performed every 24-48 h until the fascia is approximately 2-4 cm apart, and then the fascia is closed primarily [15,32,33].
The fascial closure techniques avoid the loss of domain resulting from wall retraction in OA.Achieving a reversible and tension-free TAC with facilitating reoperations is the greatest advantage of the FCTs, especially for patients with less opportunity of definitive closure of open abdomen within the first week [34,35].The primary closure time has been extended to 50 days with FCTs [36]. However, these techniques are associated with high cost and require special equipment that is not available for all surgeons. Suturing the mesh graft to the abdominal fascia may increase the risk of fascia trauma and necrosis. In addition, FCTs do not prevent formation of adherences between anterior abdominal wall and the viscera, limiting abdominal wall mobilization for primary closure.
The most significant drawbacks of FCTs include lower rate of primary closure (18-38%), and high fistula rate (7-26%) with early use of absorbable material. Use ofnonabsorbable material has improved primary closure rate (33 to 89%),however, fistula rate remained high (6-18%) [37,38]. Nowadays, the Wittmann Patch (WP) which isa nonabsorbable mesh using biological compatible artificial material, still has a popularityandisusually applied in clinical practice withoverall good outcomes [38].The primary closure rate forthe WP method ranges from 78 to 100% with lower fistula rate (0-4.2%) [39,40].
Negative-pressure therapy
The negative pressure therapy has been used in the management of OA in 1995[41], and several systems have been described. Vacuum-assisted closure technique consists of covering the bowel by the omentum underlying the wound. Then tailored polyvinyl alcohol and gelatin sponge composite material is sutured to abdominal fascia providing more accommodation of the abdomen content and preventing viscera desiccation. Next, a biological membrane is set to seal the foam and wounds (3-4 cm over the edge of incision), and a negative pressure (45-60 mmHg) is applied by connecting the silicone tube to machine. So, the abdominal cavity is separated from outside environment preventing infection.The Vacuum-assisted closure is the most commonly used technique to manage OA with various choices including Abdominal Dressing System and ABThera System [30,42].
The abdominal dressing consists of using an inert plastic encased sponge, the perforated plastic interface covers the entire viscera , paracolic gutters (right and left) and the entire fascial defect , preventing viscera adherence to the overlying peritoneum, protecting bowel and allowing fluid drainage. A macroporousGranuFoam sponge as the middle layer is fixed to the fascia and subcutaneous tissue and must not be in contact with underlying viscera and should contain drains to provide suction. Finally, a bio-occlusive adhesive sheet (Ioban) fixed laterally to the flank skin, maintains the abdominal wall integrity facilitating change of patient position if necessary.
The ABThera system (figure 1) usesa visceral protective layer covering the whole abdominal contents from pelvis to diaphragmand laterally theparacolic gutters, allowing prevention of visceraladherencesand facilitating further abdominal wall mobilisation.The protected spongeor the second layer can be placed in thepelvic spaceand deeply in the paracolic gutters resulting in facilitatingeffectiveevacuation of the peritoneal fluids. Finally an occlusive layer with GranuFoam anddraining set is applied as previously described [43]. As illustrated in figure 1, theABTherasystem allows protectionof the abdominal cavitycontent and skinfacilitatingabdominal closure.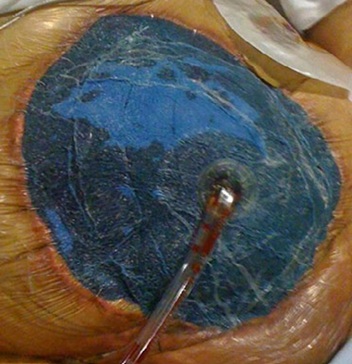 Figure 1:ABTheraTM System. The GranuFoam of the second layer can be fixed to the skin to prevent direct contact of skin edges with the negative pressure foam.
Figure 1:ABTheraTM System. The GranuFoam of the second layer can be fixed to the skin to prevent direct contact of skin edges with the negative pressure foam.
Various pressures with continuous or intermittent suction can be applied [35,44,45].These vacuum-assisted closure systemsprevent desiccation and mechanical damage of viscera with reducing fistula formation, avoid abdominal cavity contamination, reduce the abdominal domain loss with maintaining IAP and allowing evacuation of peritoneal fluids [46,47]. Also, continuous vacuum drainage is benefit to alleviating inflammation and edema, as well as facilitating wound healing.
The primary fascial closureand fistula rates using these systems were33 to 100% (average 67%) and 0-15% (average 2.9%)respectively [10,39,44,48].The fistula risk was increased with intra-abdominal sepsis, prolonged closure time and when primary closure was not possible [49].The highest primary fascia closure rates (80%) were obtained when the vacuum assisted systems were used in combination withfascial tension technique. The combined use of fascial suture placement with sequential tightening or replacement to achieve fascial approximation, and vacuum assisted techniques wasresulted in achieving a very high primary fascia closure (>86%) [35,44,45].
As reported, the negative pressure closure system is used earlier after the first operation, and FCT is often employed during the first re-exploration if closure is not anticipated in a timely fashion [9].This sequence option reduces the ACS rates during thehigh risk period of active resuscitation and facilitates evacuating alarge volume of peritoneal fluid.
The FCT increases the chances of primary closure during the subcute period. Uncomplicated patients have generally a high primary closure rate and closure can be achieved within 4-7 days regardless of TCAtype used [10,50,51].The abdominal closure timetends to be superior to one week, generally to 20-40 days with lower closure ratein patients with complicated and prolonged resuscitation efforts and hospital courses[35,45, 51-,55].Several risk factors have been identified to predict prolonged or complicated course with decreased primary closure rate including prolonged OA time, multiple injuries with particularly colonic or duodenal injury, and active infection [9,49,51].The infections (surgical site or blood stream infections) resulted in augmenting closure time and decreasing closure rate [56].Also, conservative fluid resuscitation, less blood transfusionand negative fluid balance were associated with improved rate of primary closure[49,50,51,56].
MANAGEMENT OF PATIENTS WITH AN OPEN ABDOMEN (MEDICAL TREATMENT)
The resuscitation must be continued in the postoperative setting following DCS and OA strategy. Efforts should be focused on correcting tissue oxygen delivery, coagulopathy, hypothermia and providing an energy support within first 24 h [57]. As proved, the very large volume resuscitation with overload fluids increased the incidence of ACS and OA [48]. So, damage control resuscitationwith crystalloid infusion restrictionandearly use of blood productshas increasingly improved outcomes, reduced ACS incidenceand increased early abdominal closure rate [49-51].
Additionally, recent study reports have showed a relationship between crystalloid restriction or negative fluid balance and primary abdominal closure rates [23,52].Intra-abdominal pressure (IAP) should be monitored in patients who received large -volume resuscitation for persistent hemodynamic instability or prolonged bleeding and in patients with OA secondary to ACS. Because IAH or ACS can occur or recur [53,54]. Increased IAP exceeding 20 mmHg should be monitored hourly, if any sign of organ dysfunction occurs, the TAC should be removed and replaced with a large dressing. Extending the initial incision should be considered if necessary [9].The neuromuscular blockade has been used for early management of IAH and ACS and resulted in decreasing IAP , however, the IAP returned to baseline levels once the paralysis were off [53,54].The scarce study results were controversial regarding the primary abdominal closure rate. However, a short course of neuromuscular blockadeagents (NMBAs) as adjunctto negative pressure devices and methodsmay decrease fascial edge retraction [49,55]. The high rates of NMBAs associated complications and the poorresults have limited the neuromuscular blockade use [56].The benefitsof early enteral nutrition in trauma or postoperative setting has clearly beenhighlighted andvalidatedby the several published studies[57,58].The OA results in significant protein loss (2g/day) making necessary theintroductionof nutritional support[59].Early nutrition is associated with increasedprimary abdominal closure and decrease of intestinal fistula, infection, ICU stay lengthand hospital costs [60-62].The enteral feeding is highly privileged and recommended, and should be providedthrough enteral access via nasogastric or nasojejunal feeding tube. However, gastrostomy or jejunostomytubesshould be used with caution owing to the leak and fistula risks, and potential compromise of future abdominal closure options [63,64].
CONCLUSION
Damage control surgery combined with open abdomen strategy and damage control resuscitation has resulted in improvement of outcomes and almost eradication of postoperative ACS development in severely injured patients. However open abdomen is morbid condition and several techniques have been described to minimize the associated complications and increase abdominal fascia closure rate.Themost commonly used technique is vacuum assisted closure techniquewith high rate ofabdominal fascial closure and lower complication rate. Additionally, the combined use of fascial closure technique and vacuum assisted systems wasresulted in achieving a very high primary fascia closure.
REFERENCES
- Chovanes J, Cannon JW, Nunez TC (2012) The evolution of damage control surgery. Surg Clin North Am 92:859-875.
- Moore EE (1996) Thomas G OrrMemorial Lecture. Staged laparotomy for the hypothermia, acidosis, and coagulopathy syndrome. Am J Surg172:405-410
- Rotondo MF, Zonies DH (1997) The damage control sequence and underlying logic. Surg Clin North Am 77:761-777.
- Raeburn CD, Moore EE, Biffl WL, Johnson JL, Meldrum DR, et al. (2001) The abdominal compartment syndrome is a morbid complication of postinjury damage control surgery. Am J Surg 182:542-546
- Balogh Z, McKinley BA, Cocanour CS,Kozar RA, Valdivia A, et al. (2003) Supranormal trauma resuscitation causes more cases of abdominal compartment syndrome. Arch Surg 138:637-642.
- Z hou JC, Zhao HC, Pan KH, Xu QP (2011) Current recognition and management of intra-abdominal hypertension and abdominal compartment syndrome among tertiary Chinese intensive care physicians. J Zhejiang UnivSci B 12:156-162.
- Wise R, Roberts DJ, Vandervelden S, Debergh D, De Waele JJ, et al. (2015) Awareness and knowledge of intra-abdominal hypertension and abdominal compartment syndrome: results of an international survey.Anaesthesiol Intensive Ther 47:14-29.
- Kirkpatrick AW, Roberts DJ, De Waele J,Jaeschke R, Malbrain ML, et al. (2013) Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med39: 1190-206.
- Campbell A, Chang M, Fabian T, Franz M, Kaplan M, et al. (2009) Management of the open abdomen: from initial operation to definitive closure. Am Surg 75:S1-S22.
- Barker DE, Green JM, Maxwell RA, Smith PW, Mejia VA, et al. (2007) Experience with vacuum-pack temporary abdominal wound closure in 258 trauma and general and vascular surgical patients. J Am CollSurg 204:784-792.
- Aydin C, Aytekin FO, Yenisey C, Kabay B, Erdem E, et al. (2008) The effect of different temporary abdominal closure techniques on fascial wound healing and postoperative adhesions in experimental secondary peritonitis. Langenbecks Arch Surg 393:67-73.
- Schecter WP, Ivatury RR, Rotondo MF, Hirshberg A (2006) Open abdomen after trauma and abdominal sepsis: a strategy for management. J Am CollSurg 203:390-396.
- Hirshberg A, Wall MJ Jr, Mattox KL (1994) Planned reoperation for trauma: a two year experience with 124 consecutive patients. J Trauma 37:365-369
- Garcia-Sabrido JL, Tallado JM, Christou NV,Polo JR, Valdecantos E (1988) Treatment of severe intra-abdominal sepsis and/or necrotic foci by an ‘open-abdomen’ approach: zipper and zipper-mesh techniques. Arch Surg 123:152-156.
- Wittmann DH, Aprahamian C, Bergstein JM (1990) Etappenlavage: advanced diffuse peritonitis managed by planned multiple laparotomies utilizing zippers, slide fastener, and Velcro analogue for temporary abdominal closure. World J Surg 14:218-226.
- De Waele JJ, Leppäniemi AK (2011) Temporary abdominal closure techniques. Am Surg 77:S46-S50.
- Zhang LY (2009) Application of damage control laparotomy. J Traumatic Surg 11:1-3.
- Zhang LY. Intra-abdominal volume increment: a new method for abdominal surgery. Chin J Dig Surg 10:6-8.
- Rotondo MF, Schwab CW, McGonigal MD, Phillips GR 3rd, Fruchterman TM, et al (1993) ‘Damage control’: an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma 35:375-382.
- Roberts DJ, Bobrovitz N, Zygun DA, Ball CG, Kirkpatrick AW, et al. (2015) Indications for use of damage control surgery and damage control interventions in civilian trauma patients: a scoping review. J Trauma Acute Care Surg78:1187-1196.
- Birkmeyer JD, Reames BN, McCulloch P, Carr AJ, Campbell WB, et al. (2013) Understanding of regional variation in the use of surgery. Lancet382:1121-1129.
- Watson JJ, Nielsen J, Hart K,Rikanth P, Yonge JD, et al. (2017) Damage control laparotomy utilization rates are highly variable among level I trauma centers: pragmatic, randomized optimal platelet and plasma ratios findings. J Trauma Acute Care Surg82:481-488.
- Balogh Z, McKinley BA, Holcomb JB, Miller CC, Cocanour CS, et al. (2003) Both primary and secondary abdominal compartment syndrome can be predicted early and are harbingers of multiple organ failure. J Trauma 54:848-859
- Rutherford EJ, Skeete DA, Brasel KJ (2004) Management of the patient with an open abdomen: techniques in temporary and definitive closure. CurrProblSurg 41:811-876.
- Vertrees A, Greer L, Pickett C, Nelson J, Wakefield M, et al. (2008) Modern management of complex open abdominal wounds of war: a 5-year experience. J Am CollSurg 207:801-809.
- Acosta S, Bjarnason T, Petersson U, Pålsson B, Wanhainen A, et al. (2011) Multicentre prospective study of fascial closure rate after open abdomen with vacuum and mesh-mediated fascial traction. Br J Surg 98:735-743.
- Vertrees A, Kellicut D, Ottman S, Peoples G, Shriver C (2006) Early definitiveabdominal closure using serial closure technique on injured soldiers returning from Afghanistan and Iraq. J Am CollSurg202:762-772.
- Weinberg JA, George RL, Griffin RL, Stewart AH, Reiff DA, et al. (2008) Closing the open abdomen: improved success with Wittmann Patch staged abdominal closure. J Trauma 65:345-348.
- Tieu BH, Cho SD, Luem N, Riha G, Mayberry J, et al. (2008) The use of the Wittmann Patch facilitates a high rate of fascial closure in severely injured trauma patients and critically ill emergency surgery patients. J Trauma 65:865-870.
- Cothren CC, Moore EE, Johnson JL, Moore JB, Burch JM, et al. (2006) One hundred percent fascial approximation with sequential abdominal closure of the open abdomen. Am J Surg192:238-242.
- Diaz JJ Jr, Cullinane DC, Dutton WD, Jerome R, Bagdonas R, et al. (2010) The management of the open abdomen in trauma and emergency general surgery. Part 1. Damage control. J Trauma 68:1425-1438.
- Nagy KK, Fildes JJ, Mahr C, Roberts RR, Krosner SM, et al. (1996) Experience with three prosthetic materials in temporary abdominal wall closure. Am Surg 62:331-335.
- Wittmann DH (2000) Staged abdominal repair: development and current practice of an advanced operative technique for diffuse suppurative peritonitis. ActaChirAustriaca 32:171-178.
- Boele van Hensbroek P, Wind J, Dijkgraaf MG,Busch OR, Goslings JC (2009) Temporary closure of the open abdomen: a systematic review on delayed primary fascial closure in patients with an open abdomen. World J Surg 33:199-207.
- Brock WB, Barker DE, Burns RP (1995) Temporary closure of open abdominal wounds: the vacuum pack. Am Surg 61:30-35.
- Fernandez L, Norwood S, Roettger R, Wilkins HE 3rd (1996) Temporary intravenous bag silo closure in severemabdominal trauma. J Trauma 40:258-260.
- Sammons A, Delgado A (2016) In vitro pressure manifold distribution evaluation of ABThera active abdominal therapy, V.A.C. abdominal dressing system, and the Barker’s vacuum packing technique, conducted under dynamic conditions. SAGE Open Med4: 1-4.
- Suliburk JW, Ware DN, Balogh Z, McKinley BA, Cocanour CS, et al (2003) Vacuum-assisted wound closure achieves early fascial closure of open abdomens after severe trauma. J Trauma 55:1155-1160.
- Miller PR, Meredith JW, Johnson JC, Chang MC (2004) Prospective evaluation of vacuum-assisted fascial closure after open abdomen: planned ventral hernia rate is substantially reduced. AnnSurg 239:608-614.
- Barker DE, Kaufman HJ, Smith LA, Ciraulo DL, Richart CL, et al. (2000) Vacuum pack technique of temporary abdominal closure: a 7-year experience with 112 patients. J Trauma 48:201-206.
- Regner JL, Kobayashi L, Coimbra R (2012) Surgical strategies for management of the openabdomen. World J Surg 36: 497-510.
- Teixeira PG, Salim A, Inaba K, Brown C, Browder T, et al. (2008) A prospective look at the current state of open abdomens. Am Surg 74:891-897.
- Stone PA, Hass SM, Flaherty SK, DeLuca JA, Lucente FC, et al (2004) Vacuum-assisted fascial closure for patients with abdominal trauma. J Trauma 57:1082-1086.
- Miller RS, Morris JA Jr, Diaz JJ Jr,Herring MB, May AK (2005) Complications after 344 damage-control open celiotomies. J Trauma 59:1365-1371.
- Hadeed JG, Staman GW, Sariol HS,Kumar S, Ross SE (2007) Delayed primary closure in damage control laparotomy: the value of the Wittmann Patch. Am Surg 73:10-12.
- Vogel TR, Diaz JJ, Miller RS, May AK, Guillamondegui OD, et al. (2006) The open abdomen in trauma: do infectious complications affect primary abdominal closure? Surg Infect (Larchmt) 7:433-441.
- Wyrzykowski AD, Feliciano DV (2008) Trauma damage control.In: Feliciano DV, Mattox KL, Moore EE (eds) Trauma, 6th, McGraw-Hill, New York, pp. 851-870.
- Balogh ZJ, Lumsdaine W, Moore EE, Moore FA (2014) Postinjury abdominal compartment syndrome: from recognition to prevention. Lancet 384:1466-1475.
- Cotton BA, Guy JS, Morris JA Jr, Abumrad NN (2006) The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock 26:115-121.
- Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, et al. (2007) Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma 62:307-310.
- Fouche Y, Sikorski R, Dutton RP (2010) Changing paradigms in surgical resuscitation. Crit Care Med 38:S411-S420.
- Cotton BA, Au BK, Nunez TC, Gunter OL, Robertson AM, et al. (2009) Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J Trauma 66:41-48.
- Malbrain ML, Cheatham ML, Kirkpatrick A, Sugrue M, Parr M, et al. (2006) Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. I. Definitions.Intensive Care Med 32:1722-1732.
- Cheatham ML, Malbrain ML, Kirkpatrick A, Sugrue M, Parr M, et al. (2007) Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. II. Recommendations.Intensive Care Med 33:951-962.
- Abouassaly CT, Dutton WD, Zaydfudim V, Dossett LA, Nunez TC, et al. (2010) Postoperative neuromuscular blocker use is associated with higher primary fascial closure rates after damage control laparotomy. J Trauma 69:557-561.
- Murphy GS, Vender JS (2001) Neuromuscular-blocking drugs: use and misuse in the intensive care unit. Crit Care Clin 17:925-942.
- Moore FA, Feliciano DV, Andrassy RJ, McArdle AH, Booth FV, et al. (1992) Early enteral feeding, compared with parenteral, reduces postoperative septic complications: the results of a meta-analysis. Ann Surg 216: 172-183.
- Kudsk KA, Croce MA, Fabian TC, Minard G, Tolley EA, et al. (1992) Enteral versus parenteral feeding: effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg 215:503-511.
- Cheatham ML, Safcsak K, Brzezinski SJ, Lube MW (2007) Nitrogen balance, protein loss, and the open abdomen. Crit Care Med 35:127-131.
- Collier B, Guillamondegui O, Cotton B, Donahue R, Conrad A, et al. (2007) Feeding the open abdomen. JPEN J Parenter Enteral Nutr 31:410-415.
- Dissanaike S, Pham T, Shalhub S,Warner K, Hennessy L, et al. (2008) Effect of immediate enteral feeding on trauma patients with an open abdomen: protection from nosocomial infections. J Am CollSurg 207: 690-697.
- Byrnes MC, Reicks P, Irwin E (2010) Early enteral nutrition can be successfully implemented in trauma patients with an open abdomen. Am J Surg 199:359-362.
- Smith BP, Adams RC, Doraiswamy VA, Nagaraja V, Seamon MJ, et al. (2010) Review of abdominal damage control and open abdomens: focus on gastrointestinal complications. J Gastrointest Liver Dis 19:425-435.
- Schrag SP, Sharma R, Jaik NP, Seamon MJ, Lukaszczyk JJ, et al. (2007) Complications related to percutaneous endoscopic gastrostomy (PEG) tubes: a comprehensive clinical review. J Gastrointest Liver Dis 16:407-418.
Citation: Boukerrouche A (2019) Open Abdomen and Temporarily Abdominal Closure. J Emerg Med Trauma Surg Care 6: 034
Copyright: © 2019 Abdelkader Boukerrouche, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

