
Therapeutic Possibilities in Patients with Refractory Angina Pectoris: Spinal Cord Stimulation
*Corresponding Author(s):
Vaskova JDepartment Of Medical And Clinical Biochemistry, Faculty Of Medicine, Pavol Jozef Šafárik University, Trieda SNP, Košice, Slovakia
Tel:+421 556323232,
Email:janka.vaskova@upjs.sk
Abstract
Refractory angina pectoris is defined as angina resistant to optimal medical treatment and standard coronary revascularisation procedures. Epidural Spinal Cord Stimulation (SCS) provides relief from symptoms of Refractory Angina Pectoris (RAP), but its mechanism of action remains incompletely understood. This paper provides a short description of the current possibilities in the use of neuromodulation for the treatment of refractory angina pectoris. SCS therapy has a positive influence on myocardial ischemia tolerance, absolute myocardial perfusion reserve and endothelium-mediated vasomotor function in refractory angina pectoris. Research indicates that this therapy can alleviate myocardial perfusion abnormalities in advanced coronary artery diseases.
Keywords
ABBREVIATIONS
CABG: Coronary Artery Bypass Graft;
CHD: Congenital Heart Defects;
CNS: Central Nervous System;
CPT: Cold Pressor Test;
ECG: Electrocardiography;
ECP: External Counterpulsation;
IABC: Intra-Aortic Balloon Counterpulsation;
ICD: Implantable Cardioverter Defibrillator;
IHD: Ischemic Heart Disease;
IPG: Implantable Pulse Generator;
MBF: Myocardial Blood Flow;
MI: Myocardial Infarction;
NYHA: New York Heart Association functional class;
PET: Positron Emission Tomography;
RAP: Refractory Angina Pectoris;
SCS: Spinal Cord Stimulation;
SENS: Subcutaneous Electrical Nerve Stimulation;
TENS: Transcutaneous Electrical Nerve Stimulation.
INTRODUCTION
Refractory Angina Pectoris (RAP) is defined as a chronic condition caused by clinically reversible myocardial ischemia injury in Ischemic Heart Disease (IHD), which cannot be adequately handled by a combination of drug therapy, angioplasty or coronary arterial bypass [1]. Despite the improved availability of revascularisation procedures and significant advances in pharmacotherapy, RAP remains a major medical problem with high incidence and prevalence, even in developed countries. In US, for example, the prevalence of coronary artery disease is approximately 6%. There are an estimated 600000-1800000 Americans with RAP (2.5 - 8% of CHD patients) with 50 to 100,000 new cases each year [2]. According to older conservative estimates, in Europe and US there are 200,000 patients with RAP, with a prevalence of 1:10,000 and an incidence of 1:20,000 [3]. The quality of life of patients with RAP is significantly reduced either by anginal pain and significant limitation of daily activities or psychosocial stress. The majority of patients with RAP are relatively young, predominantly male, without severe reduction in left ventricular ejection fraction after Myocardial Infarction (MI), but paradoxically with only a low annual cardiac mortality (5-7%) mainly due to the low incidence of malignant arrhythmias. Characteristics of patients with refractory angina pectoris are given in table 1. Therefore, even if none of the treatment methods RAP currently has data showing its positive impact on mortality, it is the relatively low cardiac mortality and age of patients that needs to accentuate the positive effect on quality of life [4].
| Gender | 71 % male |
| Age (years) | 63.9 ± 10 |
Clinical presentation
|
8.1 ± 6 years 3.5 76 % 66 % 68 % 17 % 58 % |
Risk factors
|
61 % 39 % 28 % 21 % 14 % |
NON PHARMACOLOGICAL TREATMENT OF REFRACTORY ANGINA PECTORIS
A broad array of therapies (medical treatment, internal mammary artery implants, extracorporeal shockwave therapy, spinal cord stimulation, transmyocardial laser revascularisation, gene therapy and cell therapy) have been investigated, none of which have become mainstream [5-8]. In patients with stable angina pectoris who have not achieved adequate therapeutic control or who are intolerant to beta-blockers ranolazine and ivabradine are used in the symptomatic treatment. These drugs are also used in the treatment of refractory angina pectoris [9]. They target either well established mechanisms of ischemia, i.e., the imbalance between myocardial oxygen supply/demand and metabolic routes in the ischemic myocardium promoting more efficient energy utilization. According to current recommendations, ivabradine is indicated for the symptomatic treatment of refractory angina in patients with normal sinus rhythm, who have intolerance to beta-blockers or in combination with beta-blockers in patients with inadequately controlled angina [1]. Ranolazine is a selective inhibitor of the late sodium current that prevents pathological increase in sodium in the ischemic myocytes, thereby preventing calcium overload [10]. In a nonrandomized trial involving purely refractory angina patients, ranolazine was shown to be an effective antianginal regimen; albeit with a number of side effects that resulted in discontinuation of the drug 1 year after the initiation of treatment [11]. This evidence has been reflected in American and European guidelines where ranolazine is supported as a second line agent in patients with refractory angina despite commonly used anti-anginals such as b-blockers, calcium channel blockers and nitrates [12].
Patients with refractory angina present with recurrent episodes of angina, some of which may be both limiting and severe and experience very poor quality of life despite optimized medical treatment [13]. A number of non-pharmacological methods also have been proposed for the treatment of RAP. However, from relevant medical evidence, only three are currently recommended: External Counter-Pulsation (ECP) and two neurostimulatory methods - Transcutaneous Electrical Nerve Stimulation (TENS) and Spinal Cord Stimulation (SCS). ECP uses the principle of intra-aortic counterpulsation similar to Intra-Aortic Balloon Counterpulsation (IABC). ECP consists of three pairs of pneumatic cuffs placed on the legs, which are inflated above the systolic blood pressure during diastole and deflated in systole according to ECG synchronization (Figure 1).
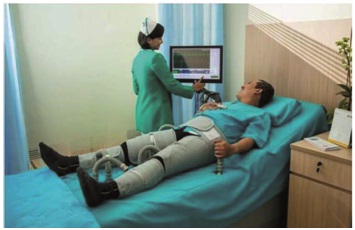
Figure 1: External Counterpulsation (ECP).
The result is an increase in diastolic blood pressure in a timely manner with increased blood flow of coronary and cerebral vasculature. It also increases venous return and decreases after load. The method is carried out 1-2 hours a day for approximately two months. This corresponds to the recommended class IIa in Europe and class IIb in USA [1,14]. TENS uses the application of low amplitude electrical stimuli through electrodes placed on the skin in the area of greatest pain (Figure 2).

Figure 2: Transcutaneous Electrical Nerve Stimulation (TENS).
The principle of action is so called "gate control", which means that the stimulation of afferent large diameter nerve fibres inhibits the input impulse transmission from small diameter fibres in the gelatinous substance of the spinal cord. The advantage of this method is its unpretentiousness, non-invasiveness and the minimal incidence of serious adverse effects. TENS can be used as a trial method prior to the planned introduction of SCS in order to determine whether myocardial ischemia is the real cause of a patient's pain. According to the European recommendations from 2013, TENS 2013 belongs to class IIb of recommendations [1].
Spinal Cord Stimulation (SCS) is an electrical neuromodulation therapy and it appears to be an effective and safe treatment option for this specific group of patients [15,16]. Several studies have shown a significant improvement in the clinical status of patients in terms of physical capacity and overall quality of life. It is likely that besides the analgesic effect of spinal stimulation, oxygen delivery to the myocardium plays an important role [4].
SPINAL STIMULATION IN REFRACTORY ANGINA PECTORIS
The basic principle on affecting the pain is based on electrical stimulation of the rear corners of the ganglia, which leads to complex activation of descending inhibitory mechanisms of CNS [15,17]. A number of theories delving deeper into the mechanism of action for spinal stimulation during RAP suggest that the presence of a spinal stimulator pronounces inhibitory effects on the cardiac sympathetic nervous system. This leads to a positive influence on myocardial blood flow in terms of redistribution ensuring correction of the imbalance between the myocardial requirement for oxygen supply and oxygen delivery in ischemic parts of the myocardium [18]. Spinal stimulation is expected to release of certain substances on the spinal level active dampening. The release of enkephalins, endorphins, blocking the painful transmission in the spinal cord is expected. It is also envisaged the mechanism of action according to Melzak “Gate control theory”, at which the facilitation of fast leading nerve fibers A and optionally fibers B occurs. A meta-analysis published by Taylor et al., revealed similar outcomes and lower healthcare costs with SCS as compared to coronary artery bypass grafting and percutaneous myocardial laser revascularization for the treatment of RAP [19]. The results of randomised clinical trials have shown promising results in terms of positive effects on symptoms. In terms of mortality, however, SCS have not shown direct positive results in the treatment of RAP, like other methods [20]. Recently published results of a randomized controlled trial RASCAL in which the effect of SCS and standard therapy were compared with the effects of standard RAP therapy alone for a period of six months. The statistical power of the study, however, was weakened through the randomisation of 29 patients instead of the planned 45. Despite this, there was a positive trend for improvement in the SCS group in terms of reduced incidence of AP, improved exercise capacity and improved quality of life [21]. According to the current European and American recommendations for the treatment of RAP based on studies provided, the SCS method belongs to class IIb (level of evidence B or C) [17].
PATIENT SELECTION
The selection of suitable patients for implantation with SCS in RAP is defined by the recommendation of the European Society of Cardiology and accessibility guidelines (Figure 3).
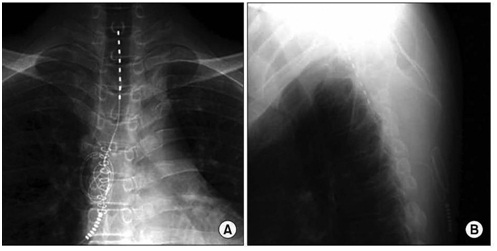 Figure 3: Chest X-ray after the introduction of the SCS electrode. The tip of electrode is in the C7-T1 epidural space (A) AP – projection; (B) side view.
Figure 3: Chest X-ray after the introduction of the SCS electrode. The tip of electrode is in the C7-T1 epidural space (A) AP – projection; (B) side view.
Patients were selected with angiographically documented coronary artery impairment, signs of myocardial ischemia, symptoms which cannot adequately handle combination drug therapy, angioplasty and coronary artery bypass graft. Contraindication for SCS implantation is that for the implantation of a spinal stimulator, such as age over 80 years, left ventricular ejection fraction lower than 40%, acute coronary syndrome, heart failure with decompensation, ventricular fibrillation or sustained ventricular tachycardia documented for other three months, severe bronchial asthma, 2nd-3rd grade AV-block and pregnancy [17]. Spinal stimulator implantation is performed most often with the intervention of an anaesthesiologist or neurosurgeon, who has been trained in the technique of implantation under the control of X-ray C–arm. The upper terminal electrodes are normally placed in the level of C7 or Th1. After generating adequate paraesthesia, the second step involved implanting a pulse generator in the case of conventional stimulators with IPG. A currently new element is the "wireless" stimulator, where implantation of the generator is not required and the second phase of the implant is not necessary. However, there is yet minimal experience with this technology.
POST-IMPLANTATION MONITORING
Protocols for patient monitoring post-implantation are not uniform and may vary depending on workplace. It is important to emphasize that, as in the use of spinal cord stimulants for the treatment of pain syndromes in post laminectomy syndromes, it is necessary to ensure quality and multidisciplinary collaboration between cardiologists, cardiothoracic surgeons and specialists in spinal cord stimulation, who are mostly specialists in algesiology. Post-implantation tracking protocol usually begins with an initial set-up of SCS and system shutdown for two weeks. After this interval, myocardial flow (Myocardial Blood Flow - MBF) is evaluated by Positron Emission Tomography (PET) in the room during an adenosine stress test and during CPT (Cold Pressor Test). It is advisable to investigate myocardial ischemic tolerance by dobutamine stress echocardiography. Of course, there should also be a questionnaire evaluating the quality of life. After analysing the above parameters without stimulation, the SCS system is activated and after 2-3 weeks of continuous stimulation, progress is determined by the same parameters. There is a standard approach to use conventional SCS for management of refractory angina with parameters of frequency most often in the range 60 - 160 Hz, pulse width 50-400 microseconds and voltage 1.5 - 6 mA. The signal to activate SCS is chest pain caused by angina.
There are some experimental cases with high frequency systems (frequency 10,000 Hz), results are not available yet. Finally, comparing the results of the examination before and after switching, SCS evaluates the effect of spinal stimulation [22].
DISCUSSION
Patients with RAP face serious medical problems despite the increase in revascularisation procedures and significant advances in the pharmacotherapy. The use of spinal stimulation in the treatment of RAP is one of the most effective solutions affecting the patient's symptoms [23]. Neuromodulation methods represent completely reversible curative methods with minimal incidence of adverse events. Electrode migration is the most common complication. Percutaneous SCS electrodes have a propensity to migrate longitudinally and more frequent than paddle type ones. Risk of infection is associated with the surgical procedure. Other complications such as electrode fracture, extension wire or implantable pulse generator failures, cerebrospinal fluid leakage, pain over the stimulator and spinal epidural hematoma, could appear [24-26].
It is appropriate to pay attention only to experts in selected centres with the necessary skills, experience and technical equipment. It is almost certain that neuromodulation will find a definite place in cardiology due to the development of neuromodulation therapy and the progress of its development, almost comparable with the development of computer technology.
Interesting, and certainly not negligible, results have been obtained from monitoring workplaces employing techniques of subcutaneous electrode implantation in the chest at the point of anginal pain so called Subcutaneous Electrical Nerve Stimulation (SENS). Preliminary results are comparable to epidural implanted systems in much less technically sophisticated technology implantation; however, long-term results of this method are absent [27].
CONCLUSION
Chronic refractory angina is a clinical diagnosis based on the presence of stable angina pectoris caused by myocardial ischemia due to advanced coronary disease. A number of techniques are used in the treatment of this condition, with an essential element being the modulation of pain. Therapeutic procedures are based on a multidisciplinary approach in which each step is decided through consultation between cardiologists, cardiac surgeons, anaesthesiologists and pain management anaesthesiologists. One of the most effective therapies for alleviating angina is the introduction of a spinal stimulator, followed by SCS. A meta-analysis of randomised trials examining this specific question brought the overall positive result for the use of SCS in RAP [19]. An evaluation of the contribution of this methodology for the group of patients still requires preparation of further clinical studies with larger groups of patients.
REFERENCES
- Task Force Members, Montalescot G, Sechtem U, Achenbach S, Andreotti F, et al (2013) 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 34: 2949-3003.
- Povsic TJ, Broderick S, Anstrom KJ, Shaw LK, Ohman EM, et al. (2015) Predictors of long-term clinical endpoints in patient with refractory angina. J Am Heart Assoc 4: 001287.
- Mukherjee D, Bhatt DL, Roe MT, Patel V, Ellis SG (1999) Direct myocardial revascularization and angiogenesis--how many patients might be eligible?. Am J Cardiol 84: 598-600.
- DeJongste MJL, Tio RA, Foreman RD (2004) Chronic therapeutically refractory angina pectoris. Heart 90: 225-230.
- Leon MB, Kornowski R, Downey WE, Weisz, G, Baim DS, et al. (2005) A blinded, randomized, placebo-controlled trial of percutaneous laser myocardial revascularization to improve angina symptoms in patients with severe coronary disease. J Am Coll Cardiol 46: 1812-1819
- Henry TD, Satran D, Jolicoeur EM (2014) Treatment of refractory angina in patients not suitable for revascularization. Nat Rev Cardiol 11: 78-95.
- McNab D, Khan SN, Sharples LD, Ryan JY, Freeman C, et al. (2006) An open label, single-centre, randomized trial of spinal cord stimulation vs. percutaneous myocardial laser revascularization in patients with refractory angina pectoris: the SPiRiT trial. Eur Heart J 27: 1048-1053.
- Weisz G, Généreux P, Iñiguez A, Zurakowski A, Shechter M, et al. (2016) Ranolazine in patients with incomplete Revascularisation after Percutaneous Coronary Intervention (RIVER-PCI): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 387: 136-145.
- Fox K, Ford I, Steg PG, Tardif JC, Tendera M, et al. (2014) Ivabradine in stable coronary artery disease without clinical heart failure. N Engl J Med 371: 1091-1099.
- Belardinelli L, Shryock JC, Fraser H (2006) Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart 92: 6-14.
- Bennett NM, Iyer V, Arndt TL, Garberich RF, Traverse JH, et al. (2014) Ranolazine refractory angina registry: 1-year results. Crit Pathw Cardiol 13: 96-98.
- Giannopoulos AA, Giannoglou GD, Chatzizisis YS (2016) Refractory angina: new drugs on the block. Expert Rev Cardiovasc Ther 14: 881-883.
- Poppi NT, Gowdak LH, Dourado LO, Adam EL, Leite TN, et al. (2017) A prospective study of patients with refractory angina: outcomes and the role of the high-sensitivity troponin T. Clin Cardiol 40: 11-17.
- Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, et al. (2014) 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 64: 1929-1949.
- de Jongste MJ, Hautvast RW, Hillege HL, Lie KI (1994) Efficacy of spinal cord stimulation as adjuvant therapy for intractable angina pectoris: a prospective, randomized clinical study Working Group on Neurocardiology. J Am Coll Cardiol 23: 1592-1597.
- Lee SH, Jeong HJ, Jeong SH, Lee HG, Choi JI, et al. (2012) Spinal cord stimulation for refractory angina pectoris – a case report. Korean J Pain 25: 121-125.
- Saraste A, Ukkonen H, Varis A, Vasankari T, Tunturi S, et al. (2015) Effect of spinal cord stimulation on myocardial perfusion reserve in patients with refractory angina pectoris. Eur Heart J Cardiovasc Imaging 16: 449-455.
- Linderoth B, Foreman RD (1999) Physiology of spinal cord stimulation: review and update. Neuromodulation 2: 150-164.
- Taylor RS, De Vries J, Buchser E, Dejongste MJ (2009) Spinal cord stimulation in the treatment of refractory angina: systematic review and meta-analysis of randomised controlled trials. BMC Cardiovasc Disord 9: 13.
- Tsigaridas N, Naka K, Tsapogas P, Pelechas E, Damigos D (2015) Spinal cord stimulation in refractory angina. A systematic review of randomized controlled trials. Acta Cardiol 70: 233-243.
- Eldable S, Thomson S, Duarte R, Brookes M, deBelder M, et al. (2016) The effectiveness and cost–effectiveness of spinal cord stimulation for refractory angina (RASCAL Study): A pilot randomized controlled trial. Neuromodulation 19: 60-70.
- Deer TR, Raso LJ (2006) Spinal cord stimulation for refractory angina pectoris and peripheral vascular disease. Pain Physician 9: 347-352.
- Simpson EL, Duenas A, Holmes MW, Papaioannou D, Chilcott J (2009) Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: systematic review and economic evaluation. Health Technol Assess 13: 1-154.
- North RB, Recinos VR, Attenello FJ, Shipley J, Long DM (2014) Prevention of percutaneous spinal cord stimulation electrode migration: a 15-year experience. Neuromodulation 17: 670-676.
- Gazelka HM, Freeman ED, Hooten WM, Eldrige JS, Hoelzer BC, et al. (2015) Incidence of clinically significant percutaneous spinal cord stimulator lead migration. Neuromodulation 18: 123-125.
- Bendersky D, Yampolsky C (2014) Is spinal cord stimulation safe? A review of its complications. World Neurosurg 82: 1359-1368.
- Cameron T (2004) Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg 100: 254-267.
Citation: Ko?an L, Rap?an R, Burianek M, Ko?anová H , Sabol F, et al. (2017) Therapeutic Possibilities in Patients with Refractory Angina Pectoris: Spinal Cord Stimulation. J Emerg Med Trauma Surg Care 4: 019.
Copyright: © 2017 Kocan L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

