
A Comparison of Visual Outcomes of Deep Anterior Lamellar Keratoplasty versus Penetrating Keratoplasty in Patients with Keratoconus
*Corresponding Author(s):
H Dwight CavanaghDepartment Of Ophthalmology, University Of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, TX 75390-9057, United States
Tel:+1 2146483770,
Fax:+1 2146489061
Email:Dwight.Cavanagh@UTSouthwestern.edu
Abstract
Methods: Retrospective chart review of 61 eyes with KCN that underwent PKP or DALK from July 2011 to January 2017. Outcome measures noted were: Best Corrected Visual Acuity (BCVA), Uncorrected Visual Acuity (UCVA), post-operative refraction, topographic astigmatism, use of Rigid Gas Permeable (RGP) lenses, and complications.
Results: Twenty six eyes were in the DALK group (42.6%), thirty five in the PKP group (57.4%). UCVA for the DALK group was 0.65 logMAR (SD=0.32) and for the PKP group 0.84 logMAR (SD=0.37) (p-value 0.043). BCVA for the DALK group was 0.205 logMAR (SD=0.16) and for the PKP group was 0.316 (SD=0.24) (p-value 0.05). The DALK group was more likely to have BCVA better or equal to 20/40 (23/26, 88.4%) than the PKP group (20/35, 57.1%), p-value 0.011. Manifest astigmatism in the DALK group was 3.69 D (SD=2.80) and in the PKP group was 3.66 D (SD=2.65), p-value 0.81. Topographic astigmatism in the DALK group was 4.13 D (SD=2.70), in the PKP group was 5.71 D (SD=4.82), p-value 0.22. Forty percent in the PKP group required post-operative RGP lens correction versus 19.2% in the DALK group, (p=0.08). There were 26.9% (7/26) complications in the DALK group versus 54.3% (19/35) in the PKP group (p-value 0.04).
Conclusion: DALK appears to provide a more favorable outcome for the surgical management of keratoconus with lower complications, including post-operative rejection rates. Although not statistically significant, DALK appears to result in better visual outcomes and lower dependence on RGP lens wear.
Keywords
INTRODUCTION
In advanced stages, disease progression in KCN leads to significant loss of the quality of vision due to corneal irregularity. Progressive protrusion can result in spontaneous breaks in Descemet’s Membrane (DM) causing acute corneal hydrops which can lead to corneal scarring [2].
The pathogenesis is not fully understood, although there have been some conditions associated with KCN such as atopy, Down’s syndrome, and eye-rubbing [3-6]. The reported incidence ranges from 1.3-25 per 100,000 per year across different populations, and a prevalence of 8.8-229 per 100,000 depending on the geographic location of the population being studied [7]. Recently, new developments have been established to improve the visual prognosis in patients with KCN using new contact lens designs in Rigid Gas Permeable Lenses (RGP CL) and piggy-back lens systems and Intracorneal Ring Segments (ICRS) [2]. Implantation of the intracorneal rings has been shown to alter the keratometry readings and improve vision [8,9].
However, the most important new therapeutic intervention that has been shown to alter the natural course of the disease is corneal cross-linking. Cross-linking was recently approved by the Food and Drug Administration in April, 2016 for progressive keratoconus and post-refractive corneal ectasia [10]. It is shown to halt the progression of the disease and stabilize or improve vision by studies investigating short and long term outcomes [11,12]. Recently, Hashemi et al., reported that over five years, cross linking stabilized Uncorrected Visual Acuity (UCVA), refraction, anterior and posterior corneal elevation, corneal power and thickness, and ultimately long term best corrected visual acuity [13]. In very advanced KCN cases, the diseased cornea may be an unsuitable candidate for these rehabilitative, new options and corneal transplantation techniques may need to be considered.
Penetrating Keratoplasty (PKP) is the transplantation of a full thickness corneal graft. It was first successfully performed by Eduard Zirm in 1905 [14]. Over the next century, surgeons began to perform PKPs using Zirm’s technique, which became the standard of care in the transplantation community [15]. Anton Elschnig performed the first anterior lamellar keratoplasty in 1914 for a case of interstitial keratitis. However, it was not until the 1950’s that Charles Tillet performed the Endothelial Keratoplasty (EK) for a case of corneal edema [15]. Given the problems that arose with lamellar keratoplasty such as interface haze and scarring, the procedure was not frequently utilized. In the 1990’s the possibility of lamellar keratoplasty was reinvestigated and reintroduced to clinical practice [16].
Deep Anterior Lamellar Keratoplasty (DALK), a form of anterior lamellar keratoplasty, involves the removal of the central corneal stroma leaving behind the host’s endothelium and DM. It is beneficial in patients with abnormal stroma and in the presence of normal endothelium, particularly in the second eye of patients with a history of endothelial rejection following PKP in the first eye. DALK is a technically more challenging procedure than PKP with the rate limiting step being successful bearing of the DM. This dissection adds an element of unreliability to the procedure. The most common intra-operative complication reported is DM perforation, which occurs in 11.7% of attempted DALK procedures [15]. Numerous techniques have been described in the literature to avoid this complication such as the Melles manual dissection, viscoelastic dissection and the Anwar big bubble technique [16-18]. The advantages of DALK over PKP based on the literature are reduced risk of endothelial rejection, lower loss of endothelial cell density, lower risk of ruptured globe injury after trauma, a potential shorter length of time of steroid use and shorter time to suture removal [15].
The purpose of this study is to compare the visual and surgical outcomes of these two surgical procedures in patients with advanced keratoconus and to compare the need for Rigid Gas Permeable (RGP) lens correction to achieve BCVA following both procedures.
METHODS AND OUTCOME MEASURES
Inclusion criteria included patients with 12 months of follow-up postoperatively. DALK successfully completed were included in the DALK group, if DALK proved infeasible intraoperatively, it was included as PKP. There were no other complications in either group. Exclusion criteria included any other pre-existing ocular pathology that would affect the best corrected visual acuity such as glaucoma, pseudophakia, aphakia and all DALK cases that needed to convert to PKP intraoperatively.
All DALK procedures were performed using the Anwar big bubble technique (100%) and the graft subsequently sutured using 16 10-0 nylon interrupted sutures. PKP was performed in the standard established technique, using running or interrupted sutures. All patients from the PKP group were instructed to use prednisolone acetate 1% on an hourly basis while awake for one month, followed by six times daily for two more months, while patients from the DALK group were instructed to use prednisolone acetate 1% on an hourly basis while awake for a two-week period, followed by six times daily for six weeks. A slow taper over a minimum of six months followed for patients from both groups according to the clinical picture. All patients had all sutures in place at time of post-operative evaluation.
Data collection points included age at the time of surgery, sex, and eye laterality. The following reported exam findings, recorded at 12 months post operatively, were included: Uncorrected Visual Acuity (UCVA), Best Corrected Visual Acuity (BCVA) with or without a need for RGP, topographic astigmatism, post-operative astigmatism on manifest refraction, and Rigid Gas Permeable (RGP) lens wear. UCVA and BCVA were compared after converting the values to logMAR values. Other outcome measures investigated were secondary high intra-ocular pressure and rejection rates (epithelial, stromal or endothelial). Pre-operative visual acuity values were extremely desperate due to differences in RGP lens wear prior to surgery. All cases included had VA of 20/80 or worse.
For statistical analysis of quantitative data, the unpaired t-test was used. For analysis of rejection rates and complication rates in both groups, a fisher’s exact test was used. Statistical significance was defined with p<0.05.
RESULTS
Sixty-one eyes met the inclusion criteria including 26 eyes in the DALK group (42.6%) and 35 eyes in the PKP group (57.4%). The patient demographics are reported in table 1. No patients had or developed infectious keratitis. The DALK patients achieved statistically significant better UCVA as well as mean Best Corrected Visual Acuity (BCVA) than the PKP group, p-value 0.04 and p-value 0.03 respectively. The DALK group was also more likely to have BCVA 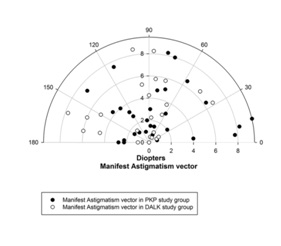
Figure 1A: Astigmatism vector in PKP group.
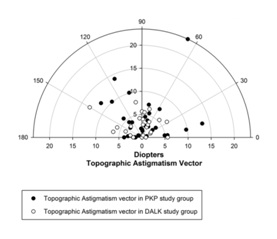
Figure 1B: Astigmatism vector in DALK group.
|
|
DALK |
PKP |
|
Number of eyes |
26 |
35 |
|
Ratio of female : Male |
1.17 |
1.92 |
|
Mean age |
35.1 |
37 |
|
|
DALK |
PKP |
P-value |
||
|
Mean |
Standard Deviation |
Mean |
Standard Deviation |
||
|
Uncorrected visual acuity (logMAR) |
0.65 |
0.32 |
0.84 |
0.37 |
0.04 |
|
Best corrected visual acuity (logMAR) |
0.21 |
0.16 |
0.32 |
0.24 |
0.03 |
|
Manifest astigmatism (Diopters) |
3.69 |
2.8 |
3.66 |
2.65 |
0.81 |
|
Topographic astigmatism (Diopters) |
4.13 |
2.7 |
5.71 |
4.82 |
0.19 |
|
|
Number and Percentage in DALK group |
Number and Percentage in PKP group |
P-Value |
|
Rigid gas permeable use |
5/26 (19.2%) |
14/35 (40%) |
0.08 |
|
Complication rate |
7/26 (26.9%) |
19/35 (54.3%) |
0.04 |
|
Best corrected visual acuity |
23/26 (88.4%) |
20/35 (57.1%) |
0.01 |
Seven patients (26.9%) in the DALK group and 19 patients (54.3%) in the PKP group had complications (p-value 0.04). Complication in both groups included development of secondary glaucoma requiring medical or surgical therapy, cataracts or dehiscence of the graft requiring re-suturing, and graft rejection (Table 3).
|
|
DALK |
PKP |
p-value (Fisher’s exact test) |
|
Rejection |
2 (all stromal) |
10 (all endothelial) |
0.0547 |
|
Secondary glaucoma |
3 |
12 |
0.0696 |
|
Cataracts |
1 |
3 |
0.6294 |
|
Dehiscence requiring re-suturing |
2 |
2 |
1 |
Two patients (8.3%) in the DALK group had stromal rejection and ten patients (28.5%) in the PKP group had endothelial rejection (Table 3).
DISCUSSION
Most of the DALK procedures in this study were performed by cornea fellows in training (23/26, 88.4%) highlighting that this procedure can be successful in the hands of less experienced surgeons.
Asena et al., reported that 17% of the eyes that underwent PKP in their series needed RGP CL fitting [24]. Another large study reported that 47% of eyes required RGP CL fitting after PKP, on an average of 8.5 months post-surgery [25]. In another study, 60% wore either bispheric or hard contact lens post PKP [26]. Prazeres et al., looked at 38 eyes post PKP and 1 eye post DALK and reported that CL fitting was done as early as 9 months but that the average time was 50.9 months, may be due to delayed referral by physicians. In that series, thirty eight percent of eyes required an RGP CL [27].
A novel finding in this study was that fewer patients in the DALK group (19.2%) required post-operative RGP lenses to improve BCVA compared to the PKP group (40%). This may be attributed to the better UCVA results post-operatively and highlights DALK as a more favorable surgical option for visual rehabilitation especially for those who grew RGP lens intolerant during the course of their disease; however, the rate of RGP use in the PKP versus the DALK group, although higher, did not meet statistical significance. We hypothesize that this could be attributed to the comparable astigmatism values in both groups. The astigmatism was 3-4 D in both groups, similar to other studies in the literature [15]. One systemic review looking at 965 eyes that underwent DALK and 2402 eyes that underwent PKP, found that patients undergoing DALK had less astigmatism as compared to PKP, but PKP had better best corrected and uncorrected visual outcomes at 6 months [21].
In agreement with the previously published studies, the PKP group had a statistically significant higher post-operative complication rate than the DALK group [15,19,23]. In the PKP group, out of the 19 eyes with complications, three eyes required cataract surgery, twelve eyes with secondary glaucoma required medical and/or surgical therapy, ten eyes were treated for rejection, and two eyes with dehiscence necessitated-suturing. In the DALK group, out of the seven eyes with complications, two eyes were treated for stromal graft rejection, three eyes needed drops for secondary glaucoma, one eye required cataract surgery, and two eyes required re-suturing, one due to dehiscence and the other due to Descemet’s Membrane detachment with a double chamber requiring re-bubbling. The complication rates in this paper are higher than the average reported in the literature. This can be attributed in part to the largely indigent, non-compliant nature of the patient population included in this study. Morever, all of the surgeries were performed at a teaching institution by surgeons with variable levels of experience.
Moreover, with regards to the complication rates in both groups, the PKP group had a higher rate of rejection than the DALK group. It can be explained by the advantages that the DALK procedure has to offer [15,22]. Firstly, the DALK procedure allows the native endothelium to remain intact hence removing the element of immune rejection of donor corneal endothelium. Secondly, as evidenced by numerous large meta-analyses, the rate of endothelial cell density loss is much higher in PKP eyes [28,29]. This is thought to be due to cell migration, instrumental manipulation, trauma to the tissue during surgery and a biphasic postoperative accelerated loss. Whether the accelerated loss of endothelial cells post-PKP is a subclinical immune endothelial rejection or a result of the other causes discussed, it has yet to be investigated further [15,30-32].
The rejection rate in this study is higher in the PKP group than in the DALK group which is in agreement with the literature. Moreover, the rejection rate in the DALK group is lower than what has been cited in recent literature. Gonzalez et al., showed an overall 14% rejection rate in DALK patients over an 18 month period with a 7-week median of postoperative steroid therapy. Thus, their conclusion was a recommendation of postoperative steroid therapy for longer than 7 weeks. Our study had postoperative steroid therapy for a minimum of six months and an 8.3% stromal rejection rate. Our results are in support of the findings of Gonzalez et al., and a longer course of postoperative steroid therapy is recommended to ensure lower rejection rates.
Extended steroid therapy carries inherent risks which include accelerated cataract formation, elevated intraocular pressure levels that may necessitate glaucoma medications or filtration surgery, ultimately driving the complication rates even higher. Thus clinical judgment must be exercised when determining the appropriate length of steroid therapy and further investigation is needed to establish a safe postoperative regimen.
This study presents the usual limitations that come with a retrospective analysis. Endothelial cell density was not available in this study which would have allowed better analysis over time and should be included in future studies. Moreover, DALK is a rarely performed procedure, which limited the number of eyes in each group and ultimately the power of the study. The rate of conversion from DALK to PKP, which reflects DALK’s most common intraoperative complication, was not included. Future studies should include larger groups to confirm the results obtained and further examine at the lower need for post-surgical RGP lenses in eyes that underwent DALK.
In conclusion, this study showed that, despite its steep learning curve, DALK appears more favorable for the surgical management of keratoconus with a lower post-operative complication rate, including rejection rates. Although not statistically significant, it allows better visual outcomes, creating a lesser need for the use of post-surgical RGP lenses to provide BCVA.
CONFLICT OF INTEREST
FUNDING
REFERENCES
- Gomes JA, Tan D, Rapuano CJ, Belin MW, Ambrósio R Jr, et al. (2015) Global consensus on keratoconus and ectatic diseases. Cornea 34: 359-369.
- Parker JS, van Dijk K, Melles GR (2015) Treatment options for advanced keratoconus: A review. Surv Ophthalmol 60: 459-480.
- Weed KH, MacEwen CJ, Giles T, Low J, McGhee CN (2008) The Dundee University Scottish Keratoconus study: demographics, corneal signs, associated diseases, and eye rubbing. Eye (Lond) 22: 534-541.
- Bawazeer A, Hodge W, Lorimer B (2000) Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol 84: 834-836.
- Wagner H, Barr JT, Zadnik K (2007) Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study: methods and findings to date. Cont Lens Anterior Eye 30: 223-232.
- Haugen OH (2009) Keratoconus in the mentally retarded. Acta Ophthalmol (Copenh) 70: 111-114.
- Liu H, Chen Y, Wang P, Li B, Wang W, et al. (2015) Efficacy and Safety of Deep Anterior Lamellar Keratoplasty vs. Penetrating Keratoplasty for Keratoconus: A Meta-Analysis. PLoS One 10: 0113332.
- Lyra JM, Lyra D, Ribeiro G, Torquetti L, Ferrara P, et al. (2017) Tomographic Findings After Implantation of Ferrara Intrastromal Corneal Ring Segments in Keratoconus. J Refract Surg 33: 110-115.
- Torquetti L, Ferrara G, Almeida F, Cunha L, Araujo LP, et al. (2014) Intrastromal corneal ring segments implantation in patients with keratoconus: 10-year follow-up. J Refract Surg 30: 22-26.
- Food and Drug Administration (2018) Approved Drug Products with Therapeutic Equivalence Evaluations (38thedn). Food and Drug Administration, Silver Spring, Maryland, USA.
- O'Brart DP, Patel P, Lascaratos G, Wang VK, Tam C, et al. (2015) Corneal Cross-linking to Halt the Progression of Keratoconus and Corneal Ectasia: Seven-Year Follow-up. Am J Ophthalmol 160: 1154-1163.
- Raiskup F, Theuring A, Pillunat LE, Spoerl E (2015) Corneal collagen crosslinking with riboflavin and ultraviolet-A light in progressive keratoconus: ten-year results. J Cataract Refract Surg 41: 41-46.
- Hashemi H, Seyedian MA, Miraftab M, Fotouhi A, Asgari S (2013) Corneal collagen cross-linking with riboflavin and ultraviolet a irradiation for keratoconus: long-term results. Ophthalmology 120: 1515-1520.
- Zirm EK (1989) Eine erfolgreiche totale Keratoplastik (A successful total keratoplasty). 1906. Refract Corneal Surg 5: 258-261.
- Reinhart WJ, Musch DC, Jacobs DS, Lee WB, Kaufman SC, et al. (2011) Deep anterior lamellar keratoplasty as an alternative to penetrating keratoplasty a report by the american academy of ophthalmology. Ophthalmology 118: 209-218.
- Sugita J, Kondo J (1997) Deep lamellar keratoplasty with complete removal of pathological stroma for vision improvement. Br J Ophthalmol 81: 184-188.
- van Dooren BT, Mulder PG, Nieuwendaal CP, Beekhuis WH, Melles GR (2004) Endothelial cell density after deep anterior lamellar keratoplasty (Melles technique). Am J Ophthalmol 137: 397-400.
- Anwar M, Teichmann KD (2002) Big-bubble technique to bare Descemet’s membrane in anterior lamellar keratoplasty. J Cataract Refract Surg 28: 398-403.
- MacIntyre R, Chow SP, Chan E, Poon A (2014) Long-term outcomes of deep anterior lamellar keratoplasty versus penetrating keratoplasty in Australian keratoconus patients. Cornea 33: 6-9.
- Yüksel B, Kandemir B, Uzunel UD, Çelik O, Ceylan S, et al. (2017) Comparison of visual and topographic outcomes of deep-anterior lamellar keratoplasty and penetrating keratoplasty in keratoconus. Int J Ophthalmol 10: 385-390.
- Henein C, Nanavaty MA (2017) Systematic review comparing penetrating keratoplasty and deep anterior lamellar keratoplasty for management of keratoconus. Cont Lens Anterior Eye 40: 3-14.
- Trimarchi F, Poppi E, Klersy C, Piacentini C (2001) Deep Lamellar Keratoplasty. Ophthalmologica 215: 389-393.
- Keane M, Coster D, Ziaei M, Williams K (2014) Deep anterior lamellar keratoplasty versus penetrating keratoplasty for treating keratoconus. Cochrane Database Syst Rev 22: CD009700.
- Asena L, Alt?nörs DD (2016) Visual Rehabilitation After Penetrating Keratoplasty. Exp Clin Transplant 14: 130-134.
- Geerards AJ, Vreugdenhil W, Khazen A (2006) Incidence of rigid gas-permeable contact lens wear after keratoplasty for keratoconus. Eye Contact Lens 32: 207-210.
- Smiddy WE, Hamburg TR, Kracher GP, Stark WJ (1988) Keratoconus. Contact lens or keratoplasty? Ophthalmology 95: 487-492.
- Prazeres S, Malet F, Colin J (2008) Contact lens fitting after keratoplasty for keratoconus. J Fr Ophtalmol 31: 849-854.
- Borderie VM, Sandali O, Bullet J, Gaujoux T, Touzeau O, et al. (2012) Long-term results of deep anterior lamellar versus penetrating keratoplasty. Ophthalmology 119: 249-255.
- Kubaloglu A, Sari ES, Unal M, Koytak A, Kurnaz E, et al. (2011) Long-term results of deep anterior lamellar keratoplasty for the treatment of keratoconus. Am J Ophthalmol 151: 760-767.
- Reinhard T, Bohringer D, Hüschen D, Sundmacher R (2002) [Chronic endothelial cell loss of the graft after penetrating keratoplasty: influence of endothelial cell migration from graft to host]. Klin Monbl Augenheilkd 219: 410-416.
- Bourne WM (2001) Cellular changes in transplanted human corneas. Cornea 20: 560-569.
- Bohringer D, Reinhard T, Godehardt E, Sundmacher R (2001) [Regression analysis of idiopathic endothelial cell loss after perforating normal risk keratoplasty: basic principles for long-term analysis of endothelial risk factors in a retrospective clinical study]. Klin Monatsbl Augenheilkd 218: 412-417.
Citation: Mahasneh S, Saade JS, Abiad B, Cavanagh HD (2018) A Comparison of Visual Outcomes of Deep Anterior Lamellar Keratoplasty versus Penetrating Keratoplasty in Patients with Keratoconus. J Ophthalmic Clin Res 4: 045.
Copyright: © 2018 Shaam Mahasneh, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

