
Journal of Ophthalmology & Clinical Research Category: Clinical
Type: Case Report
Branch Retinal Artery Occlusion Associated with Hyperhomocysteinemia: Case Report
*Corresponding Author(s):
Sellami DorraMedicine, Medicine University, Sfax, Tunisia
Tel:+216 98519893,
Fax:+216 74400818
Email:dorra_sellami@yahoo.fr
Received Date: Dec 05, 2014
Accepted Date: Jan 13, 2015
Published Date: Jan 27, 2015
Abstract
Introduction
Elevated plasma homocysteine is a risk factor for cardiovascular, cerebrovascular and peripheral vascular disease. We report the case of a young patient with branch ocular artery occlusion due to hyperhomocysteinemia.
Patients and methods
A19-year-old patient without significant medical history, presented for sudden, painless perception of black shadow in superior visual field of right eye since one day. Ophthalmologic examination revealed default hemi upper visual field in the right eye, best-corrected visual acuity 10/10 P2 on both eyes. Anterior segment exam was normal in both eyes, while the right fundus revealed normal disc with white ischemic retina encroaching the foveolar avascular zone around the infero-temporal vascular. Fluorescein angiography confirmed delayed filling of the right branch retinal artery occlusion.
Results
A work-up was performed, including: a full lipid profile, serum electrolytes, erythrocyte sedimentation rate, C reactive protein, a complete blood count (leukocytes, platelets, hemoglobin were normal), a coagulation work-up (prothrombin time/partial thromboplastin time, protein C, protein S, antithrombin III, factor V Leiden were normal), c-antineutrophil cytoplasmic antibody, antiphospholipid antibodies and antinuclear antibodies were negative, and finally cardiology studies (cardiac echo, carotid Doppler) and neurology (brain MRI) were ordered and came back normal. Otherwise, plasma homocysteine was moderately high, at 21.66 μmol/L. The patient was treated with platelet aggregation inhibitors and vitamin supplements and the condition was successfully controlled.
Conclusion
High plasma homocysteine is a risk factor for retinal vascular occlusive disease so it may be useful to measure homocysteine in the management of these patients.
Keywords
Branch retinal arterial occlusion; Homocysteine; Hyperhomocysteinemia; Vitamin B12
INTRODUCTION
Retinal vascular diseases occur rarely in young patients. More than 90% of cases occur in people older than the age of 50 years and as many as 70% of patients have arterial hypertension, cardiovascular atherosclerotic disease, or diabetes mellitus [1]. Elevated plasma homocysteine is a recently recognized vascular risk factor for thrombosis and vascular diseases [2]. It is also shown to be a significant risk factor for retinal vascular occlusions [2]. Here we describe a young man with occlusion of branch retinal artery associated with homocysteinaemia.
CASE REPORT
A19-year-old patient without significant medical history, presented with sudden, painless and permanent perception of black shadow in superior visual field of right eye since one day. Ophthalmologic examination revealed default hemi upper visual field in the right eye, best-corrected visual acuity 10/10 P2 on both eyes. Anterior segment examination was unremarkable with normal pupillary reactions. Intraocular pressures were 12 and 13 mmHg OD and OS with applanation tonometry, respectively. Fundus examination revealed normal disc with white ischemic retina encroaching the foveolar avascular zone around the infero-temporal vascular in the right eye (Figure 1). Ophthalmologic examination was totally normal in the left eye. Fluorescein angiography showed a delay in the filling of inferotemporal artery in the right eye and capillary non-perfusion corresponding to the area of retinal whitening seen clinically (Figure 2). Visual field examination showed a default of the hemi upper visual field in the right eye (Figure 3). He was diagnosed as a branch retinal artery occlusion. There was no history for any sign of autoimmune diseases. The patient's family history was negative for thrombotic events. Physical examination and chest X-ray revealed no evidence of malignant disease or any other pathology. Haematological and cardiological consultation together with some laboratory tests (Table 1) including complete blood count, serum lipids and cholesterol, prothrombin time/partial thromboplastin time, erythrocyte sedimentation rate, fibrinogen, complement factors C3 and C4, antithrombin III, protein C activity, protein S antigen, activated protein C resistance, folate, vitamin B6, serum vitamin B12 levels , rheumatoid factor, antinuclear antibodies, c-Antineutrophil Cytoplasmic Antibody (cANCA), antiphospholipid antibodies and the factor V Leiden, were ordered and came back normal. Otherwise, the serum homocysteine level was found to be 21.66 µmol/l (normal range: 0-12 µmol/l).
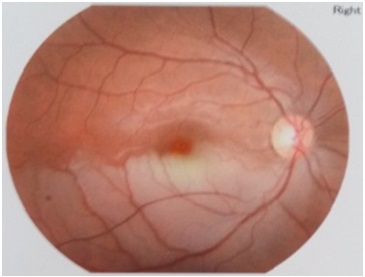
Figure 1: Fundus examination revealed retinal oedema, and whitening in the inferior temporal arcuate in the right eye.
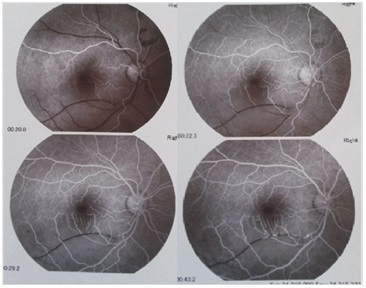
Figure 2: Fluorescein angiography showing a delay in the filling of inferotemporal artery in the right eye.
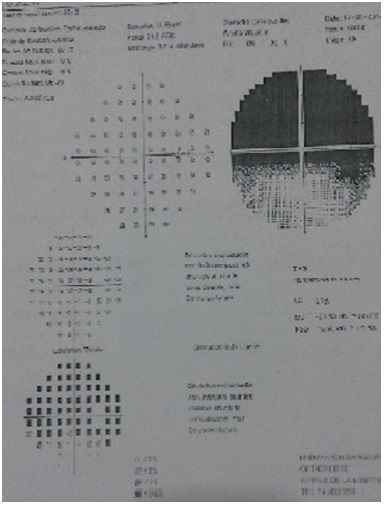
Figure 3: Visual field examination showed a default of the hemi upper visual field in the right eye.
| Complete blood count |
| Serum lipids and cholesterol |
| Prothrombin time/partial thromboplastin time |
| Erythrocyte sedimentation rate |
| Fibrinogen |
| Complement factors C3 and C4 |
| Antithrombin III |
| Protein C activity |
| Protein S antigen |
| Activated protein C resistance |
| Folate |
| Vitamin B6 |
| Serum vitamin B12 |
| Rheumatoid factor |
| Antinuclear antibodies |
| c-Antineutrophil Cytoplasmic Antibody (cANCA) |
| Antiphospholipid antibodies |
| Factor V Leiden |
The patient was treated with platelet aggregation inhibitors and vitamin supplements (vitamin B1: 300 mg/day, vitamin B6: 60 mg/day and vitamin E: 200 mg/day) and the condition was successfully controlled. At the subsequent visits, the white retinal edema had disappeared but the lost of the visual field persisted (Figure 4-5). The serum homocysteinemia was nearly normalized (14.52 µmol/l).
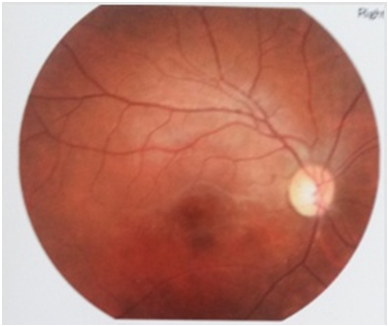
Figure 4: Fundus examination 4 months after treatment.
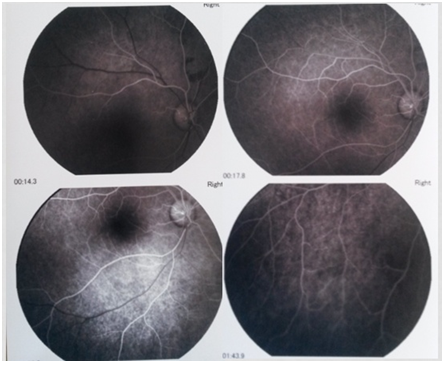
Figure 5: Fluorescein angiography 4 months after treatment.
DISCUSSION
Branch retinal artery obstruction is a rare event, even less common than central retinal artery obstruction overall. Branch retinal artery obstruction is, however, more prevalent than central retinal artery obstruction in young patients [1]. Conditions that promote embolus formation in youth include atrial myxoma, mitral valve prolapse, rheumatic heart disease, and endocarditis [1]. Youth may also develop a hypercoagulable state secondary to hyperhomocysteinemia, factor V Leiden mutations, or antiphospholipid antibody syndrome [1].
Hyperhomocysteinemia is an established risk factor for vascular diseases including retinal vascular occlusion [3]. The exact mechanism by which high plasma concentrations of homocysteine induce arterial and venous thrombosis is still not clear. Endothelial injury by release of free radicals, creating an environment of hypercoagulability, and by modification of vessel wall is probably the key mechanism of thrombotic and atherosclerotic complications [2]. Weger et al., [4] found that the mean plasma homocysteine levels were significantly higher in patients with retinal artery occlusion compared with normal controls. Vine et al., [5] described hyperhomocysteinemia as a risk factor for retinal vascular occlusion in 74 patients. Martin et al., [6], by measuring the homocysteine levels in 70 patients with retinal vascular disease, conclude that hyperhomocysteinia may be a risk factor for retinal vascular disease and could be simply and cheaply treated with folate and vitamins B6 and B12. Parchand et al., [7] found raised serum homocysteine levels in a patient presenting a primary branch retinal artery occlusion with idiopathic retinal vasculitis, aneurysms, and neuroretinitis syndrome. Furthermore, Coban-Karatas et al., [8] reported central retinal artery occlusion associated with hyperhomocysteinemia caused by methylenetetrahydrofolate reductase C677T mutation and high lipoprotein (a) level in a child. In our case, homocystinaemia was the only predisposing factor for artery occlusion. Thus, it may be useful to measure homocysteine in the management of premature retinal vascular diseases.
Differing mean plasma homocysteine values for healthy people from various countries have been reported that range from 6 μmol/L in Japan to 13 μmol/L in South Africa [9]. In 1997, Alfthan et al., [9] reported the mean plasma homocysteine concentrations in 13 different countries which ranged from 7.1 μmol/L in Germany to 10.7 μmol/L in Finland. Recent studies indicate that 15 to 30 percent of patients with premature occlusive vascular disease have moderately elevated total plasma homocysteine concentrations (higher than 15 μmol/L). A plasma homocysteine concentration exceeding 15 μmol/L defines hyperhomocysteinemia [10].
Moderate hyperhomocyteinémia (15-30 μmol/L) reflects impaired pathway of remethylation. The possible causes include deficiency of folic acid, vitamin B12 or dysfunction of 5,10-Methyltetrahydrofolate Reductase (MTHFR). A point mutation of amino acid 677 (677 C → T) in MTHFR gene can causes alanine-valine substitution and is associated with reduced enzyme activity of MTHFR. This is the commonest form of genetic hyperhomocysteinemia [11]. Severe hyperhomocysteinemia (>100 μmol/L) may be caused by deficiency of homozygote Cystathionine β-Synthase (CBS), homozygote thermo-stable MTHFR, or enzymes catalyzing vitamin B12 metabolism. Abnormal increase of plasma Hcy (>15 μmol/L) after a methionine load (100 mg/kg) may reflect impaired Hcy transsulfuration due to deficiency of heterozygous CBS or vitamin B6 [12].
Patients with retinal vascular occlusive disease have also a significant excess of mortality from coronary and cerebrovascular diseases [13]. So it is assumed that by reducing the toxic effect of homocysteine on the blood vessels, the probability of further retinal and systemic vascular occlusion, and therefore the resulting morbidity and mortality will be reduced. Randomized clinical trials have shown that oral supplementation with the combination of folate, B6-, and B12-vitmains substantially lowers circulating homocysteine levels [14]. If effective, the simplicity, availability, and presumably favorable side effect profile of hyperhomocysteinemia treatment with combined folate and oral B6- and B12-vitamin supplementation makes this an attractive addition to standard medical therapy for retinal vascular diseases and primary prevention of cardiovascular disease.
Further, some studies have demonstrated that hyperhomcysteinemia might be involved in the pathophysiology of many neuropsychiatric disorders, including brain atrophy, epilepsy, and Alzheimer’s disease [15-18]. But homocysteinemia-lowering therapies from an early age are it effective at preventing neuropsychiatric disorders? Answer to this question remains novel area for further work.
Hyperhomocysteinemia is an established risk factor for vascular diseases including retinal vascular occlusion [3]. The exact mechanism by which high plasma concentrations of homocysteine induce arterial and venous thrombosis is still not clear. Endothelial injury by release of free radicals, creating an environment of hypercoagulability, and by modification of vessel wall is probably the key mechanism of thrombotic and atherosclerotic complications [2]. Weger et al., [4] found that the mean plasma homocysteine levels were significantly higher in patients with retinal artery occlusion compared with normal controls. Vine et al., [5] described hyperhomocysteinemia as a risk factor for retinal vascular occlusion in 74 patients. Martin et al., [6], by measuring the homocysteine levels in 70 patients with retinal vascular disease, conclude that hyperhomocysteinia may be a risk factor for retinal vascular disease and could be simply and cheaply treated with folate and vitamins B6 and B12. Parchand et al., [7] found raised serum homocysteine levels in a patient presenting a primary branch retinal artery occlusion with idiopathic retinal vasculitis, aneurysms, and neuroretinitis syndrome. Furthermore, Coban-Karatas et al., [8] reported central retinal artery occlusion associated with hyperhomocysteinemia caused by methylenetetrahydrofolate reductase C677T mutation and high lipoprotein (a) level in a child. In our case, homocystinaemia was the only predisposing factor for artery occlusion. Thus, it may be useful to measure homocysteine in the management of premature retinal vascular diseases.
Differing mean plasma homocysteine values for healthy people from various countries have been reported that range from 6 μmol/L in Japan to 13 μmol/L in South Africa [9]. In 1997, Alfthan et al., [9] reported the mean plasma homocysteine concentrations in 13 different countries which ranged from 7.1 μmol/L in Germany to 10.7 μmol/L in Finland. Recent studies indicate that 15 to 30 percent of patients with premature occlusive vascular disease have moderately elevated total plasma homocysteine concentrations (higher than 15 μmol/L). A plasma homocysteine concentration exceeding 15 μmol/L defines hyperhomocysteinemia [10].
Moderate hyperhomocyteinémia (15-30 μmol/L) reflects impaired pathway of remethylation. The possible causes include deficiency of folic acid, vitamin B12 or dysfunction of 5,10-Methyltetrahydrofolate Reductase (MTHFR). A point mutation of amino acid 677 (677 C → T) in MTHFR gene can causes alanine-valine substitution and is associated with reduced enzyme activity of MTHFR. This is the commonest form of genetic hyperhomocysteinemia [11]. Severe hyperhomocysteinemia (>100 μmol/L) may be caused by deficiency of homozygote Cystathionine β-Synthase (CBS), homozygote thermo-stable MTHFR, or enzymes catalyzing vitamin B12 metabolism. Abnormal increase of plasma Hcy (>15 μmol/L) after a methionine load (100 mg/kg) may reflect impaired Hcy transsulfuration due to deficiency of heterozygous CBS or vitamin B6 [12].
Patients with retinal vascular occlusive disease have also a significant excess of mortality from coronary and cerebrovascular diseases [13]. So it is assumed that by reducing the toxic effect of homocysteine on the blood vessels, the probability of further retinal and systemic vascular occlusion, and therefore the resulting morbidity and mortality will be reduced. Randomized clinical trials have shown that oral supplementation with the combination of folate, B6-, and B12-vitmains substantially lowers circulating homocysteine levels [14]. If effective, the simplicity, availability, and presumably favorable side effect profile of hyperhomocysteinemia treatment with combined folate and oral B6- and B12-vitamin supplementation makes this an attractive addition to standard medical therapy for retinal vascular diseases and primary prevention of cardiovascular disease.
Further, some studies have demonstrated that hyperhomcysteinemia might be involved in the pathophysiology of many neuropsychiatric disorders, including brain atrophy, epilepsy, and Alzheimer’s disease [15-18]. But homocysteinemia-lowering therapies from an early age are it effective at preventing neuropsychiatric disorders? Answer to this question remains novel area for further work.
CONCLUSION
Retinal vascular occlusions in young patient should be investigated throughout for embolic sources and any disease causing thrombophilia as well as local ocular conditions. We would like to stress on the investigation for homocystinaemia as a predisposing condition for the occlusion of branch retinal artery occlusion in the absence of other risk factors. The next question to be answered is whether homocysteine -lowering therapy contributes to the prevention of retinal vascular occlusion.
REFERENCES
- Greven CM, Slusher MM, Weaver RG (1995) Retinal arterial occlusions in young adults. Am J Ophthalmol 120: 776-783.
- Welch GN, Loscalzo J (1998) Homocysteine and atherothrombosis. N Engl J Med 338: 1042-1050.
- Stanger O, WegerM, Renner W, Konetschny R (2001) Vascular dysfunction in hyperhomocyst(e)inemia. Implications for atherothrombotic disease. Clin Chem Lab Med 39: 725-733.
- Weger M, Stanger O, Deutschmann H, Leitner F J, Renner W, et al. (2002) The role of hyperhomocysteinemia and Methylenetetrahydrofolate Reductase (MTHFR) C677T mutation in patients with retinal artery occlusion. Am J Ophthalmol 134: 57-61.
- Vine AK (2000) Hyperhomocysteinemia: a risk factor for central retinal vein occlusion. Am J Ophthalmol 129: 640-644.
- Martin SC, Rauz S, Marr JE, Martin N, Jones AF, et al. (2000) Plasma total homocysteine and retinal vascular disease. Eye (Lond) 14 : 590-593.
- Coban-Karatas M, Erol I, Ozkale Y, Yazici N (2013) Central retinal artery occlusion in a 13-year-old child as a presenting sign of hyperhomocysteinemia together with high lipoprotein (a) level. Pediatr Neurol 49:138-140.
- Parchand S, Bhalekar S, Gupta A, Singh R (2012) Primary branch retinal artery occlusion in idiopathic retinal vasculitis, aneurysms, and neuroretinitis syndrome associated with hyperhomocysteinemia. Retin Cases Brief Rep 6: 349-352.
- Alfthan G, Aro A, Gey KF (1997) Plasma homocysteine and cardiovascular disease mortality. Lancet 349: 397.
- Bakker RC, Brandjes DP (1997) Hyperhomocysteinaemia and associated disease. Pharm World Sci 19: 126-132.
- Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, et al. (1995) A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10: 111-113.
- Selhub J (1999) Homocysteine metabolism. Annu Rev Nutr 19: 217-246.
- Cugati S, Wang JJ, Knudtson MD, Rochtchina E, Klein R, et al. (2007) Retinal vein occlusion and vascular mortality: pooled data analysis of 2 population-based cohorts. Ophthalmology 114: 520-524.
- Maron BA, Loscalzo J (2009) The treatment of hyperhomocysteinemia. Annu Rev Med 60: 39-54.
- Sachdev P (2004) [Homocysteine and neuropsychiatric disorders]. Rev Bras Psiquiatr 26: 50-56.
- Baldelli E, Leo G, Andreoli N, Fuxe K, Biagini G, et al. (2010) Homocysteine potentiates seizures and cell loss induced by pilocarpine treatment. Neuromolecular Med 12: 248-259.
- Sachdev PS (2005) Homocysteine and brain atrophy. Prog Neuropsychopharmacol Biol Psychiatry 29: 1152-1161.
- Religa D, Styczynska M, Peplonska B, Gabryelewicz T, Pfeffer A, et al. (2003) Homocysteine, apolipoproteine E and methylenetetrahydrofolate reductase in Alzheimer’s disease and mild cognitive impairment. Dement Geriatr Cogn Disord 16: 64-70.
Citation: Sellami D, Chaabène M, Abid F, Gargouri S , Feki J, et al. (2015) Branch Retinal Artery Occlusion Associated with Hyperhomocysteinemia: Case Report. J Ophthalmic Clin Res 2: 005.
Copyright: © 2015 Sellami Dorra, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2026, Copyrights Herald Scholarly Open Access. All Rights Reserved!

