
Journal of Brain & Neuroscience Research Category: Clinical
Type: Research Article
FGF-2 Enhances Generation of ApoE-Containing HDL along with FGF-1 in Rat Astrocytes under Oxidative Stress
*Corresponding Author(s):
Jin-ichi ItoDepartment Of Nutrition, Faculty Of Health And Nutrition, Shubun University, Ichinomiya, Japan
Tel:+81 586452101,
Fax:+81 586454410
Email:ito.j@shubun.ac.jp
Received Date: Feb 22, 2018
Accepted Date: May 01, 2018
Published Date: May 18, 2018
Abstract
The release of Fibroblast Growth Factor 1 (FGF-1) along with cytosolic proteins is enhanced from rat astrocytes under gentle oxidative stress condition using Hydrogen Peroxide (H2O2) without inducing apoptosis. FGF-1 promotes the generation of apolipoprotein E-containing High-Density Lipoprotein-like particles (apoE/HDL) in astrocytes to preserve cholesterol homeostasis and to protect neural cells from oxidative stress in the brain. In this work, we newly found that oxidative stress promotes release of FGF-2 from rat astrocytes and FGF-2 enhances apoE/HDL generation as well as FGF-1.
The treatment of rat astrocytes with 100 µM H2O2 for 10 min enhanced the release of FGF-2 as well as FGF-1 without increasing the mRNA expression of these FGFs. FGF-2 promoted not only mRNA expression and secretion of apoE but also synthesis and release of Cholesterol (Cho) in rat astrocytes like FGF-1. The apoE and Cho released from FGF-2- or FGF-1-treated rat astrocytes were recovered with the HDL fraction with densities of 1.16-1.113 g/ml, suggesting that FGF-2 enhances apoE/HDL generation. FGF-2 suppressed H2O2-induced release of HSP70 and HSP90 from rat astrocytes. This finding implies that FGF-2 has protection action on astrocytes under oxidative stress. FGF-1 raised the levels of mRNA expression and production of FGF-2 in rat astrocytes. These findings suggest that the production of FGF-2 is enhanced by FGF-1 and both FGFs upregulate apoE/HDL generation to protect astrocytes from oxidative stress.
Keywords
Astrocytes; ApoE; Cholesterol; FGF-2; FGF-1; HDL, Oxidative stress
ABBREVIATIONS
HDL: High Density Lipoprotein; BBB: Blood Brain Barrier; Apo: Apolipoprotein; DPBS: Dulbecco’s Phosphate Buffered Saline; FCS: Fetal Calf Serum; DMEM: Dulbecco’s Modified Eagle Medium; BSA: Bovine Serum Albumin; TLC: Thin Layer Chromatography; SDS-PAGE: 0.5% SDS/12.5% Polyacrylamide Gel Electrophoresis; TCA: Trichloroacetic Acid; RT-PCR: Reverse Transcription-Polymerase Chain Reaction; FGF: Fibroblast Growth Factor; LXR: Liver X Receptor; BSA: Bovine Serum Albumin; IGF1: Insulin-Like Growth Factor 1; Cho: Cholesterol; SM: Sphingomyelin; PC: Phosphatidylcholine; tBH: t-Butyl Hydroperoxide; CM: Conditioned Medium; Ins: Insulin
INTRODUCTION
As FGF-1 and FGF-2 have no N-terminal signal peptide, these growth factors are seemingly secreted by the mechanism different from the classical secretary pathway [1,2]. It is known that FGF-1 is released under various stress conditions [3-6]. However, the mechanism underlying the release is not clearly understood at present. We observed that the production and release of FGF-1 in astrocytes are enhanced by stressful long-term culture accompanied with oxidative stress [7,8]. We, furthermore, found that the treatment of rat astrocytes with 100 µM H2O2 for 10 min enhances release of FGF-1 along with cytosolic proteins without inducing apoptosis [9]. We observed previously that the apoptosis is not apparently induced in rat astrocytes treated with 100 µM H2O2 within 18 h after the commencement of H2O2 addition [9]. Furthermore, the cellular level of lactate dehydrogenase in cultured rat astrocytes did not significantly decrease in the cells treated with at 1 h after the commencement of the treatment with 300 µM H2O2. On the basis of these findings, the treatment of the cells with 100 µM H2O2 for 10 min was employed in the experiments to examine release of FGF-1 or FGF-2 without inducing apoptosis. The treatment of rat astrocytes with 100 µM H2O2 for 10 min also suppresses transiently syntheses of lipids such as Cholesterol (Cho), Phosphatidylcholine (PC), and Sphingomyelin (SM) and enhances release of these lipids from the cell surface [10], suggesting that the imbalance of plasma membrane lipids enhances release of cytosolic FGF-1 through the increase of transient permeability.The FGF-1 released from H2O2 treated rat astrocytes enhances apoE/HDL generation of stress-less astrocytes through increasing in syntheses and release of apoE and lipids [1,2]. FGF-1 appears to use multi-pathways for stimulation of apoE mRNA expression and enhancement of Cho biosynthesis in astrocytes [11,12]. The increasing biosynthesis of FGF-1 is observed in the astrocytes around the lesion of the cryo-injury in a mouse brain, followed by up-regulated apoE production [13]. It has been already known that the suppression of Cho synthesis decreases viability of neurons and addition of Cho as apoE/HDL resumes and develops synapse formation and neurite outgrowth in cultured cell systems [14]. The apoE/HDL is thought significant for neuronal development.
It is well known that astrocytes produce greatly FGF-2 as well as FGF-1 [1]. In this work, we studied whether oxidative stress induces FGF-2 release from rat astrocytes and whether FGF-2 enhances apoE/HDL generation like FGF-1 in order to understand the mechanisms underlying apoE/HDL generation and protection from neuronal stress in the brain.
MATERIALS AND METHODS
Cell culture
Astrocytes were prepared from the brain of 17-day Wistar rat fetus according to the method previously described [15]. After removal of the meninges, the brain was cut into small pieces and treated with 0.1% trypsin solution in Dulbecco’s phosphate buffered saline containing 0.15% glucose (0.1% trypsin/DPBS/G) for 3 min at room temperature. The cell pellet was obtained by centrifugation at 300 × g for 3 min and cultured in F-10 medium containing 10% fetal calf serum (10% FCS/F-10) at 37°C for 1 week as a primary culture. The cells in a primary culture were treated with 0.1% trypsin/DPBS/G containing 1 mM Ethylene Diamine Tetra Acetic Acid (EDTA), seeded at 2 × 105 cells/dish (a six-well multiple tray) in 2 ml of 10% FCS/F-10, and cultured for one week for a regular secondary culture (W/W cells). The secondary culture was carried out for one month to prepare long-term cultured rat astrocytes (W/M cells).
To induce oxidative stress, rat astrocytes (W/W and W/M cells) were washed 3 times with DPBS, incubated in 0.1% Bovine Serum Albumin (BSA)/F-10 for 24 h, and treated with or without 100 µM H2O2or t-Butyl Hydroperoxide (tBH) for 10 min. The cells were then washed 3 times with DPBS and incubated in 0.02% BSA/F-10 for the experiments.
Analysis of proteins in whole cell and Conditioned Medium (CM) by western blotting
The CM of rat astrocytes (W/W and W/M cells), from which cell debris was removed by centrifugation at 10,000 × g for 30 min, were treated with 10% Trichloroacetic Acid (TCA) to obtain a protein pellet. After SDS-PAGE(0.5% SDS/10% polyacrylamide gel) of protein pellets of the CM (released from 100 µg proteins of the cells) or the whole cells (100 µg proteins) and transfer to a Sequi-Blot TM PDVF Membrane (BIO RAD), immuno-stainning was carried out using goat anti-human FGF-1 (FGF-1 (C-19): sc-1884, Santa Cruz), rabbit anti-human FGF-2 (FGF-2 (147): sc-79, Santa Cruz), mouse anti-HSP70 (BD Biosciences), mouse anti-HSP90 (BD Biosciences), mouse anti-HSP110 (BD Biosciences),or mouse anti-?-actin antibody (SIGMA), or rabbit anti-apoE antiserum, a generous gift from Dr. Jean Vance (University of Alberta). The anti-FGF-1 antibody reacts with FGF-1 of mouse, rat and human origin and the anti-FGF-2 antibody does specifically with precursor and mature FGF-2 of mouse, rat, and human. Western blotting was carried independently at least 3 times. Representative result in each experiment was shown in each figure.
Analysis of CM by sucrose-density gradient centrifugation
After washing 3 times with DPBS, rat astrocytes were incubated with or without [14] acetate (4 µCi/ml) in 0.1% BSA/F-10 for 24 h and washed with DPBS 3 times again. The CM (7 ml) prepared from the cells (1 mg protein) cultured in a fresh 0.02% BSA/F-10 for the indicated time was over layered on 18 ml of sucrose solution with 1.172 g/ml and centrifuged at 166,400 × g for 48 h with a RP50T roter (HITACHI), after removal of cell debris by centrifugation at 10,000 × g for 30 min. The sample was separated into 12 fractions in order from the bottom of the centrifugal tube. An aliquot (500 µl) of each fraction was used for protein analysis by western blotting with specific antibody after precipitation with 10% Trichloroacetatic Acid (TCA). Alternatively, the lipid was extracted from 1 ml of each fraction with 2 ml of chloroform/methanol solution (2:1, v/v) and analyzed by Thin Layer Chromatography (TLC) on Silica Gel-60 plate (Merck) for Cho quantitative determination according to the method previously described [16].
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from rat astrocytes with ISOGEN (Wako LIFE SCIENCE), and reverse-transcripted to cDNA using a Super Script Pre-amplification System (Gibco BRL). The resulting cDNA was subjected to PCR by using the specific DNA probes for mRNA (50 ng) of rat apoE, FGF-1, FGF-2, GAPDH, ?-actin, or HMG-CoA reductase according to the previous method [4]. 5’-GCAGCATCACTTCGCTTCC-3’ (sense) and 5’-TGGAAGAAACAGTATGGCCTTCTG-3’ (antisense) were newly used for FGF-2 as primer pairs. RT-PCR was independently carried out 3 times for each experiment. Representative results were shown in each figure.
Biosynthesis and release of lipid
After the treatment with or without 100 µM H2O2 for 10 min and washed, W/W cells were incubated with [14C]-acetate (4 µCi/ml; New England Nuclei) in fresh 0.02% BSA/F-10 for 2 h for Cho synthesis. Lipid was extracted from the cells with hexane-isopropanol (3:2; v/v) and analyzed by TLC. The data points represent the mean ± SD of the measured numerical values by using three cell plates (n=3).
For lipid release assay, rat astrocytes were incubated with or without FGF-1 or FGF-2 (0 or 100 ng/ml) in the presence of [14C]-acetate (2 µCi/ml) in 0.1% BSA/F-10 for 24 h. After washing, the cells were incubated in a fresh 0.02% BSA/F-10 for 16 h. Lipid was extracted from the CM with chloroform: methanol (2:1, v/v) solvent mixture, followed by Cho analysis by TLC. Total protein amounts in W/W cells were determined and the values of Cho analysis were calibrated as [14C]-labeled Cho released from the cells (1 mg of cell protein) to the CM. The experiments were carried out using three cell plates of W/W cells (n=3).
Isolation of FGF-1, FGF-2, and apoE using Heparin-Sepharose
The CM of W/W and W/M cells (1 mg of cell protein) incubated in a fresh 0.02% BSA/F-10 for the indicated incubation time was incubated with Heparin-Sepharose (GE Healthcare) at room temperature for 2 h. The gel was washed 3 times with 0.02 M Tris/saline/protease inhibitor and analyzed by Western blotting by using a specific antibody against FGF-1, FGF-2, or apoE.
Statistical analysis
Each experiment was performed using three cell plates and repeated at least once in an independent experiment. Quantitative results are expressed as mean ± Standard Deviation (SD). Representative results were shown. Values were statistically analyzed by Student t-test, and a value of p<0.05 (*) or p<0.01 (**) was considered to be statistically significant.
RESULTS AND DISCUSSION
We showed in the previous paper that astrocytes enhance FGF-1 release under oxidative stresses, and that FGF-1 stimulates astrocytes to increase the generation of apoE/HDL which suppress the release of cytosolic proteins such as HSP70 and HSP90 [7,9,10,17]. In this work, oxidative stress induced by the treatment with 100 µM H2O2 or tBH for 10 min hardly enhanced mRNA expressions of both FGF-1 and FGF-2 regardless of the presence or absence of Fetal Calf Serum (FCS) in the media (Figure 1A). However, H2O2 enhanced the release of FGF-1 and FGF-2 along with cytosolic protein HSP90 from both W/W and long-term cultured rat astrocytes (W/M cells), although the suppression of apoE secretion (Figure 1B). The release of FGF-1 was especially enhanced from W/M cells under the oxidative stress with 100 µM H2O2. The FGF-2 release was not so much enhanced from the long-term cultured W/M cells by the treatment with H2O2 as compared with FGF-1, while FGF-2 was released more actively than FGF-1 from stress-less W/W cells by the treatment with H2O2. It appears that the long-term culture stress enhances much more the release of FGF-1 than that of FGF-2 in rat astrocytes.
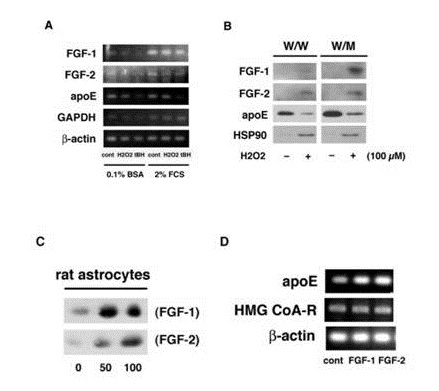
Figure 1: Effects of FGF-2 and FGF-1 on production and release of apoE. W/W cells were treated with or without 100 µM H2O2 or tBH for 10 min and washed. The cells were incubated in 0.1% BSA/F-10 or 2% FCS/F-10 for 24 h to analyze mRNA expression of FGF-1, FGF-2, apoE, GAPDH, and ?-actin (A). W/W and W/M cells were treated with or without 100 µM H2O2 and incubated in fresh 0.02% BSA/F-10 for 24 h. The CM was incubated with Heparin-Sepharose for analysis of the release of FGF-1, FGF-2, and apoE, and without Heparin-Sepharose for HSP90 by Western blotting (B). W/W cells were incubated with or without 50 or 100 ng/ml of FGF-1 (SIGMA) or FGF-2 (WAKO) for 24 hand then in a fresh 0.02% BSA/F-10 for 16 h for analysis of apoE secretion by Western blotting (C). W/W cells were treated with FGF-1 or FGF-2 (0 or 100 ng/ml) for 24 h and washed. RT-PCR was performed for the determination of mRNA expression of apoE, HMG CoA-R, and ?-actin by using specific probes (D).
It was next examined whether FGF-2 promotes apoE/HDL generation of rat astrocytes through the increase of syntheses of both apoE and Cho like FGF-1. Not only FGF-1 but also FGF-2 enhanced both secretion (Figure 1C) and mRNA expression (Figure 1D) of apoE in rat astrocytes. FGF-1 and FGF-2, furthermore, enhanced synthesis (Figure 2A) and release (Figure 2B) of Cho in/from rat astrocytes. The secretion of apoE from rat astrocytes was enhanced by FGF-2 and FGF-1, and the apoE was recovered with the HDL fraction with density of 1.16-1.113 g/ml in the CM along with Cho ((Figures 2C and 2D). These findings suggest that FGF-2 enhances apoE/HDL generation of rat astrocytes like FGF-1 through the increase in syntheses and release of both apoE and Cho.
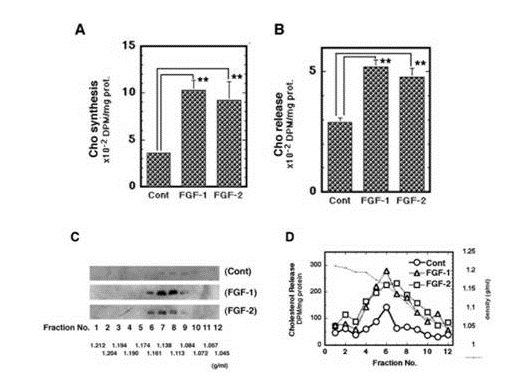
Figure 2: Effects of FGF-2 on apoE/HDL generation in rat astrocytes. W/W cells were incubated with [14C]-acetate after or before the 24 h-incubation with FGF-1 or FGF-2 for analysis of synthesis (A) or release (B) of Cho by TLC. W/W cells were treated with or without FGF-1 or FGF-2 for 24 h and incubated in fresh 0.02% BSA/F-10 for 16 h. The CM of the cells (1 mg of protein) was analyzed by sucrose-density gradient centrifugation and Western blotting for distribution of apoE (C). W/W cells treated with or without FGF-1 or FGF-2 (100 ng/ml) in the presence of [14C]-acetate (4 µCi/ml) were incubated in fresh 0.02% BSA/F-10 for 16 h. The CM prepared was analyzed by sucrose-density gradient centrifugation and TLC for released Cho (D). The experiments were carried out 2 times independently. Representative results are shown in each figure.
The H2O2 induced release of cytosolic proteins such as HSP90 and HSP70 was suppressed by the pretreatment of W/W cells with FGF-1 or FGF-2 (Figure 3A). FGF-1 and FGF-2 also suppressed the release of cytosolic proteins from W/M cells which have undergone oxidative stress during a long-term culture (Figure 3B). Thus the pretreatment with FGF-1 or FGF-2 suppressed the oxidative stress-induced release of cytosolic proteins from rat astrocytes. This finding suggests that FGF-2 is also able to protect astrocytes from oxidative stress. It is unclear in this study whether FGF-1 or FGF-2 suppresses indirectly the protein release through the increasing apoE/HDL generation or directly without participation of the apoE/HDL generation.
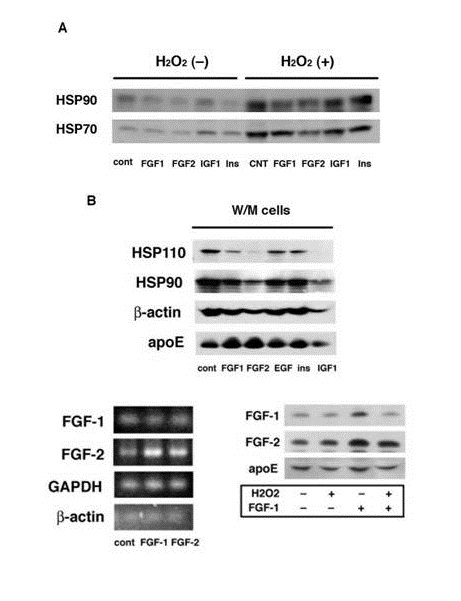
Figure 3: : Effects of FGF-1 and FGF-2 on oxidative stress-induced release of cytosolic proteins. W/W (A) or W/M cells (B) were treated with or without 100 ng/ml of FGF-1, FGF-2, insulin (Ins), (SIGMA), epidermal growth factor (EGF, SIGMA), or insulin-like growth factor 1 (IGF1, WAKO) for 24 h. W/W cells were then treated with or without 100 µM H2O2. The CM was analyzed for the release of HSP110, HSP90, HSP70, ?-actin, or apoE. Total mRNA was extracted from W/W cells treated with or without FGF-1 or FGF-2 and analyzed by RT-PCR for FGF-1, FGF-2, GAPDH, or ??actin (C). W/W cells were treated with or without 100 µM H2O2, washed, and incubated with or without 100 ng/ml of FGF-1 for 24 h. The cells were treated by sonication in 0.02M Tris buffer, pH 7.5, containing protease inhibitors cocktail (Sigma) for 20 sec, followed by centrifugation at 367,000 × g for 30 min. The supernatant obtained from 1 mg protein of cells was incubated with Heparin-Sepharose for analysis of FGF-1, FGF-2, and apoE (D).
What is the mechanism to enhance the release of FGF-2 in rat astrocytes under oxidative stress? FGF-1 enhanced the mRNA expression and production of FGF-2, although FGF-2 increased hardly those of FGF-1 (Figure 3C and 3D). The pretreatment with H2O2 suppressed the binding or incorporation of exogenously added FGF-1 with/into rat astrocytes (Figure 3D). These findings suggest a possibility that oxidative stress enhances the release of FGF-1 and then FGF-1 up-regulates FGF-2 production in oxidative stress-less astrocytes. The action of FGF-1 to enhance FGF-2 production may strengthen the function of astrocytes to generate apoE/HDL for protection of the brain from oxidative stress. Byrd et al., reported that FGF-1 enhances IL-2 production in FGF receptor-bearing Jurk at T cells. FGF-1 is greatly expressed to induce T cell infiltration in sites of chronic immunologic injury [18]. T cells stimulated with peptide growth factors such as FGFs enhance cytokine production through a second signal. FGFs seemingly activate T cells to secrete cytokine such as IL-2 in the site of immunologic injury. It is very interesting whether there is a synergistic effect between FGF-1 and FGF-2 for apoE/HDL generation and protection of neural cells from oxidative stress. The physiological relevance of increase of FGF-2 production induced by FGF-1 should be examined in astrocytes to relate to brain protection against injury and stress in vivo and in vitro in details as a next study.
CONCLUSION
In this study, we attempted to examine whether FGF-2 is actually released under oxidative stress as well as FGF-1, and whether FGF-2 enhances apoE/HDL generation in astrocytes also. The experimental results in this work suggested that gentle oxidative stress enhances the release of FGF-2, which up-regulates apoE/HDL generation as well as FGF-1, in rat astrocytes. FGF-1, furthermore, enhanced the mRNA expression and production of FGF-2 in rat astrocytes. These FGFs may participates to delay the progression of Alzheimer’s disease with oxidative stress through the increase in apoE/HDL generation, because of the protection activities of FGFs against oxidative stress.
REFERENCES
- Mason IJ (1994) The ins and outs of fibroblast growth factors. Cell 78: 547-552.
- Wiedlocha A, Nilsen T, Wesche J, Sorensen V, Malecki J, et al. (2005) Phosphorylation-regulated nucleocytoplasmic trafficking of internalized fibroblast growth factor-1. Mol Biol Cell 16: 794-810.
- Ito J, Nagayasu Y, Lu R, Kheirollah A, Hayashi M, et al. (2005) Astrocytes produce and secrete FGF-1, which promotes the production of apoE-HDL in a manner of autocrine action. J Lipid Res 46: 679-686.
- Jackson A, Friedman S, Zhan X, Engleka KA, Forough R, et al. (1992) Heat shock induces the release of fibroblast growth factor 1 from NIH 3T3 cells. Proc Natl Acad Sci U S A 89: 10691-10695.
- Shin JT, Opalenic SR, Wehby JN, Mahesh VK, Jackson A, et al. (1996) Serum-stavation induces the extracellular appearance of FGF-1. Biochim Biophys Acta 1312: 27-38.
- Mouta CC, Landriscina M, Bellum S, Prudovsky I, Maciag T (2001) The comparative release of FGF1 by hypoxia and temperature stress. Growth Factors 18: 277-285.
- Nagayasu Y, Ito J, Nishida T, Yokoyama S (2008) Reactivity of astrocytes to fibroblast growth factor 1 for biogenesis of apolipoprotein E-high density lipoprotein is down-regulated by long-time secondary culture. J Biochem 143: 611-616.
- Nagayasu Y, Morita SY, Hayashi H, Miura Y, Yokoyama K, et al. (2014) Increasing cellular level of phosphatidic acid enhances FGF-1 production in long term-cultured rat astrocytes. Brain Res 1563: 31-40.
- Ito J, Nagayasu Y, Hoshikawa M, Kato KH, Miura Y, et al. (2013) Enhancement of FGF-1 release along with cytosolic proteins from rat astrocytes by hydrogen peroxide. Brain Res 1522: 12-21.
- Ito J, Nagayasu Y, Ogawa T, Okihara H, Michikawa M (2015) Biochemical properties in membrane of rat astrocytes under oxidative stress. Brain Res 1615: 1-11.
- Ito J, Nagayasu Y, Okumura-Noji K, Lu R, Nishida T, et al. (2007) Mechanism for FGF-1 to regulate biogenesis of apoE-HDL in astrocytes. J Lipid Res 48: 2020-2027.
- Lu R, Ito J, Iwamoto N, Nishimaki-Mogami T, Yokoyama S (2009) FGF-1 induces expression of LXRalpha and production of 25-hydroxycholesterol to upregulate the apoE gene in rat astrocytes. J Lipid Res 50: 1156-1164.
- Tada T, Ito J,Asai M, Yokoyama S (2004) Fibroblast growth factor 1 is produced prior to apolipoprotein E in the astrocytes after cryo-injury of mouse brain.Neurochem Int 45: 23-30.
- Michikawa M, Yanagisawa K (1999) Inhibition of cholesterol production but not of nonsterol isoprenoid products induces neuronal cell death. J Neurochem 72: 2278-2285.
- Ito J, Kato T, Yamakawa Y, Kato H, Sakazaki Y, et al. (1982) Interaction of glia maturation factor with the glial cell membrane. Brain Res 243: 309-314.
- Ito J, Nagayasu Y, Kato K, Sato R, Yokoyama S (2002) Apolipoprotein A-I induces translocation of cholesterol, phospholipid, and caveolin-1 to cytosol in rat astrocytes. J Biol Chem 277: 7929-7935.
- Ueno S, Ito J, Nagayasu Y, Furukawa T, Yokoyama S (2002) An acidic fibroblast growth factor-like factor secreted into the brain cell culture medium upregulates apoE synthesis, HDL secretion and cholesterol metabolism in rat astrocytes. Biochim Biophys Acta 1589: 261-272.
- Byrd VM, Ballard DW, Miller GG, Thomas JW (1999) Fibroblast growth factor-1 (FGF-1) enhances IL-2 production and nuclear translocation of NF-kappaB in FGF receptor-bearing Jurkat T cells. J Immunol 162: 5853-5859.
Citation: Hoshikawa M, Yokoyama S, Hida H, Michikawa M, Ito J (2018) FGF-2 Enhances Generation of ApoE-Containing HDL along with FGF-1 in Rat Astrocytes under Oxidative Stress. J Brain Neursci 2: 002.
Copyright: © 2018 Mariko Hoshikawa, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

Journal Highlights
- Neuroscience
- Neurosurgery
- Nervous System
- Brain injury
- Brain Function
- Neuroplasticity
- Synaptic Transmission
- Neurogenetics
- Sensory Processing
- Neuropharmacology
- Motor Control
- Cognitive Impairment and Aging
- Neural Imaging Techniques
- Neuroimmunology
- Mental Health and Resilience
- Motor Imagery
- Neuro Oncology
- Dementia
- Depression
© 2026, Copyrights Herald Scholarly Open Access. All Rights Reserved!
