
Immune Genes Highly Expressed by Microglia: Roles in Physiological and Pathological Conditions in the CNS
*Corresponding Author(s):
Marianne Von Euler ChelpinDepartment Of Psychiatry And Neurochemistry, Gothenburg University, Sweden
Tel:+46 762163505,
Email:marianne.von.euler.chelpin@gu.se
Abstract
Microglia are the first line of defense in the Central Nervous System (CNS) and plays a central role in maintaining brain homeostasis. Microglia remove damaged neurons and control innate and adaptive immune responses. Additionally, major emergent activities such as influence in cognition and behaviour, have currently were described. Here we examined the database ImmGen (Immunological Genome Project) to determine which mice genes associated with the immune system had the highest expression in microglia. We found that the Colony Stimulating Factor 1 Receptor (CSF1R), Chemokine (C-C motif) Ligand 3 (CCL3), C1q Complex Protein subunits a, b and c (C1q a, b, c), CX3C Chemokine Receptor 1 (CX3CR1), Interleukin 10 receptor alpha subunit (IL-10ra), C-C Chemokine Receptor type 5 (CCR5), Interferon Gamma Receptor 2 (IFNGR2), Interleukin-10 Receptor Beta subunit (IL-10rb), C-C Chemokine Receptor-Like 2 (CCRL2), Chemokine (C-C motif) Ligand 9 (CCL9), Interleukin-4 Receptor Alpha (IL-4ra), Interleukin-1 Alpha (IL-1a), Chemokine (C-C motif) Ligand 4 (CCL4) and Chemokine (C-C motif) Ligand 6 (CCL6) are highly expressed. We provide a summary of the involvement of these molecules in homeostatic conditions and numerous neurological diseases and hope to awaken the interest to further study these genes and the networks they form in the context of the CNS.
Keywords
INTRODUCTION
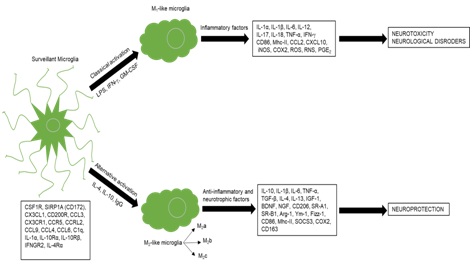
Figure 1: Representation of the polarization states of microglia.
At physiological conditions, microglia acquires a surveilling phenotype and express multiple proteins (lower left) necessary to maintain brain homeostasis. When stimulated with Lipopolysaccharide (LPS), IFN-? and Granulocyte-Macrophage Colony Stimulating-Factor (GM-CSF) microglia become classically activated and acquire an M1-like phenotype characterized by the release of multiple inflammatory factors. The release of these factors by microglia leads to neurotoxicity causing neurological disorders. In the presence of IL-4, IL-10 and Immunoglobulin G (IgG) microglia become alternatively activated with an M2-like phenotype and release multiple anti-inflammatory factors that lead to neuroprotection.
Microglial markers: Homeostasis vs. activation
A property of microglia is their rapid activation after a CNS insult which leads to an increase in cell volume, number and cluster formation [14]. Microglia take on an amoeboid shape and exhibit enhanced immunoreactivity for Iba-1 and upregulate the common leukocyte antigen Cluster of Differentiation 45 (CD45) [11]. Activated microglia also express other molecules that are involved in antigen presentation, T cell stimulation and phagocytosis. These include the major Histocompatibility Complex Class II (Mhc-II), Cluster of Differentiation 11c (CD11c, also known as integrain alpha X), Cluster of Differentiation 80 (CD80, B7-1), Cluster of Differentiation 86 (CD86, B7-2), Cluster of Differentiation 40 (CD40), Cluster of Differentiation 163 (CD163) and Cluster of Differentiation 204 (CD204) [15-18] (Figure 2). Recently, Peferoen, et al., using an in vitro microglia model, showed the existence of microglia populations expressing markers that could differentiate their phenotypes. Cluster of differentiation 74 (CD74), CD40, CD86 and C-C Chemokine Receptor Type 7 (CCR7) were found to be specific for M1-like microglia while Mannose Receptor (MR) and C-C Motif Chemokine 22 (CCL22) were specifically expressed by M2-like microglia [19].
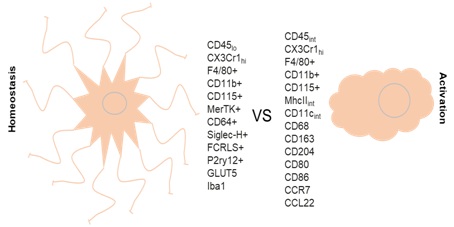
Figure 2: Microglia markers at homeostasis and after activation.
In homeostatic conditions and after activation microglia express a large number of proteins specific for each state.
Finally, it is important to mention that a consensus regarding the nomenclature of CNS-resident vs CNS-infiltrating myeloid cells has not yet been established under inflammatory conditions. A better classification and analysis of the different myeloid cells in the inflamed brain might help untangle their functions during pathological conditions.
Immune-gene expression by microglia in health and disease
We examined the first 1264 positions of the gene expression profiles corresponding to microglia (designated as MF.Microglia.CNS) in the ImmGen database [20,21] to determine which immune-related genes had the highest expression in microglia under basal conditions. Based on the results, the genes were classified into four categories.
1. Colony Stimulating Factor 1 Receptor
2. Chemokines and chemokine receptors
3. Interleukins and interleukin receptors and
4. Complement system proteins (Figure 3)
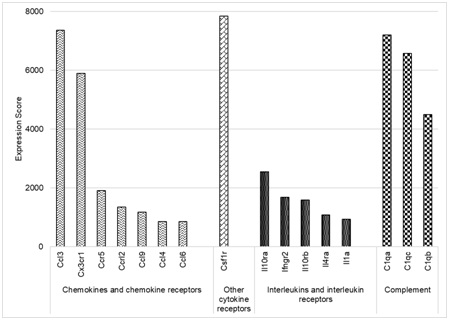
Figure 3: Schematic representation of the highest expressed immune-genes in microglia.
The highest expressed gene in our selection was CSF1R. Among the chemokines and chemokine receptors we found CCL3, CX3CR1, CCR5, CCRL2, CCL9, CCL4 and CCL6 to be highly expressed. The interleukins and interleukin receptors that had the greatest expression scores were IL-10ra, IFNGR2, IL-10rb, IL-4ra and IL-1a. Finally, genes corresponding to subunits of the complement system C1q (C1qa, C1qb and C1qc) were also highly expressed. The interleukins and interleukin receptors that had the greatest expression scores wereIL-10ra, IFNGR2, IL-10rb, IL-4ra and IL-1a. Finally, genes corresponding to subunits of the complement system C1q (C1qa, C1qb and C1qc) were also highly expressed. Together these results indicate that at physiological conditions microglia have a very high expression of immune-related genes, highlighting the importance of the immune system in maintaining brain homeostasis.
The genes were divided into four categories: other cytokine receptors (CSF1R); chemokines and chemokine receptors; interleukins and interleukin receptors and complement system.
A brief description of the functions and roles of these molecules in healthy state and neurological diseases is provided in table 1.
| Type | Other Name | Function | Disease Model | References |
| CSF1R | M-CSFR, CD115 | Regulates neuronal survival and differentiationInactivating mutations lead to progressive dementiaRegulates the activation and proliferation of microgliaProlonged inhibition resulted in the blockade of microglia proliferation and shift to anti-inflammatory phenotypeImproved performance in memory and behavioral tasks through pharmacological targetingMicroglia in the adult brain are dependent on CSF1R signaling | Alzheimer’s Disease | [22,23,24] |
| Chemokines and Receptors | ||||
| CCL3 | MIP1-α | Inflammatory chemokineRegulates migration, proliferation and cytokine expressionMediates accumulation of microgliaInduces inflammation and cognitive failure through Aβ1-40 | Neuropathic painBrain injuryAlzheimer’s Disease | [25-27] |
| CX3CR1 | Fractalkine receptor 1 | Upregulated after peripheral nerve injuryCritical for the initial development of chemotherapy-induced neuropathic painDeletion of CX3CR1 promotes recovery after spinal cord injury, induces changes in microglia function and enhances endogenous repair and neuroplasticity | Neuropathic painSpinal Cord Injury | [28,29] |
| CCR5 | Chemoattractant proteinBlockade of CCR5 downregulates expression and function of M2markers (ARG1, IL-10) and reduces microglia migrationAblation of CCR5 prevents neuronal injury and microglia activation; protects against spatial learning and memory impairmentCCR5 KO mice had less number of TH+ neurons, larger dopamine depletion, behavioral impairments and microglia activation | GlioblastomaHIV-associated brain injuryParkinson’s Disease | [30-32] | |
| CCRL2 | CCRL2 deficiency exacerbates EAE clinical phenotypesCCRL2 deficiency elevated the microglia markers Iba1, CD68 and TREM2Important player in EAE-associated inflammatory reactionsAnti-inflammatory role during chronic phase of EAE | Multiple Sclerosis/EAE | [33,34] | |
| CCL9 | MIP1-g | Pro-inflammatory chemokinePotential involvement in regulation of macrophage and microglia cellsMelatonin inhibits its expression in BV2 cells | Retinal DamageType 2 Diabetes | [35,36] |
| CCL4 | MIP1-β | Related with cell motilityExpressed by activated microglia after light damageIncrease in CCL4-CCR5signaling in spinal dorsal horn of diabetic monkeys contributes to neuroinflammation | [37,38] | |
| CCL6 | C10 | Expressed in rat microglia without stimulationMediates the migration of microgliaMediator of cell-cell communication under physiological and pathological conditions of CNSKey role in the recruitment of macrophage lineage cells to the CNSPossible role in the process of inflammatory demyelination | EAE | [39,40] |
| Interleukins and Receptors | ||||
| IL10RA | Trend to increased expression in the anterior lumbar spinal cord | Amyotrophic Lateral Sclerosis | [41] | |
| IFNGR2 | Polymorphisms in IFNGR2 allele increase susceptibility to schizophreniaTriplication of IFNGR2 increases inflammation and worsened outcome of Down’s Syndrome | Paranoid SchizophreniaDown’s Syndrome | [42,43] | |
| IL10RB | Upregulated in the high risk group of Glioblastoma patients with poor survivalUpregulated in the somatosensory cortex and olfactory bulb of neuroserpin mutated mice | GlioblastomaNeuroserpinopathy | [44,45] | |
| IL4RA | Upregulation on microglia serves to enhance their sensitivity to IL-4 and promote neuroprotective CNS environmentUpregulation is decreased in microglia of aged mice leading to a failure to induce an anti-inflammatory phenotype | [46] | ||
| IL1A | α-Syn intra-cerebral injection induces an increased expression of IL-1α in striatumUpregulated after brain damageKey mediator of sterile inflammatory response | Parkinson’s DiseaseHypoxic-ischemic brain damage | [47-49] | |
| Complement System | ||||
| C1qaC1qbC1qc | Protein levels of C1q significantly increased in refractory epilepsy samplesC1q localizes to microglia and dendritesC1q deficiency causes increased synaptic density and seizuresC1q is increased and associated with synapses in Alzheimer’s modelsNecessary for the toxic effects of soluble Aβ oligomers on synapsesSignificantly increased in sclerotic gray matter lesionsC1qa implicated in response to stimulus and stress. Central role in manifestation of schizophrenia and bipolar disorder | EpilepsyAlzheimer’s DiseaseMultiple SclerosisSchizophreniaBipolar Disorder | [50,51-53] | |
MIP: Macrophage Inflammatory Protein; ARG1: Arginase-1; EAE: Experimental Autoimmune Encephalomyelitis; TREM2: Triggering Receptor Expressed On Myeloid Cells 2; TH: Tyrosine Hydroxilase; Aβ: Amyloid-Beta Peptide; KO: Knock-Out; CD115: Cluster of Differentiation 115; CD68: Cluster of Differentiation 68; α-Syn: Alpha-Synuclein.
Cytokines and their receptors in the CNS
Upon stimulation, microglia release high levels of a vast number of pro-inflammatory factors that can cause extreme neuroimmune responses (Figure 1). Once the injury ceases the levels of inflammation are generally controlled through the release of multiple anti-inflammatory mediators, among them, IL-10 and IL-4 (Figure 1). The binding of these cytokines to their receptors activates numerous anti-inflammatory signalling cascades [60] that control fundamental steps in the immune response such as decreasing cytokine gene expression and down-regulation of Mhc-II [61].
The fact that microglia express both pro and anti-inflammatory receptors at homeostatic conditions highlights their versatility to adopt different phenotypes in response to the cellular milieu.
Chemokines and their receptors in the CNS
Our results showed that the chemokine receptor with the highest expression was CX3CR1 (also known as fractalkine receptor). CX3CR1 has been implicated in synaptic pruning of microglia in the healthy brain [69] and is regularly used for tracing microglia lineage [70]. It has been implied that signalling of CX3CR1 with its ligand, the Chemokine (C-X3-C motif) Ligand 1 (CX3CL1) regulates microglia phenotype [71]. Another highly expressed chemokine is CCL3, which has been considered a hippocampal neuromodulator capable of regulating mechanisms of synaptic plasticity involved in learning and memory functions [72]. A dysregulation of CCL3 can result in neuro inflammation, for example, there is an increased expression of this chemokine around sclerotic lesions [73,74]. Table 1 presents a description and involvement of the highest expressed chemokines and their receptors in several neurological pathologies such as Multiple Sclerosis, Alzheimer’s Disease, stroke, trauma and other [75,76].
Complement system in the CNS
FINAL REMARKS
The curated data presented here (obtained from the only one microglia set available in the ImmGen database) can help visualize the impact of microglia in the basal immunological environment present in the CNS and perhaps predict the implications of its disruption in the context of neurological diseases. We believe that once the functions of these genes in the CNS context are elucidated, it will be possible to develop molecular tools to help modulate inflammation and control adverse effects in CNS pathologies.
ACKNOWLEDGEMENT
REFERENCES
- Michell-Robinson MA, Touil H, Healy LM, Owen DR, Durafourt BA, et al. (2015) Roles of microglia in brain development, tissue maintenance and repair. Brain 138: 1138-1159.
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, et al. (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330: 841-845.
- Mittelbronn M, Dietz K, Schluesener HJ, Meyermann R (2001) Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol 101: 249-255.
- Santiago AR, Bernardino L, Agudo-Barriuso M, Gonçalves J (2017) Microglia in health and disease: a double-edged sword. Mediators of Inflammation 2.
- Perry VH (2016) Microglia. Microbiol Spectr 4.
- Gomez-Nicola D, Perry VH (2015) Microglial dynamics and role in the healthy and diseased brain: a paradigm of functional plasticity. Neuroscientist 21: 169-184.
- Ransohoff RM (2016) How neuroinflammation contributes to neurodegeneration. Science 353: 777-783.
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM (2006) Gene expression profile of activated microglia under conditions associated with dopamine neuronal damage. FASEB J 20: 515-517.
- Subramaniam SR, Federoff HJ (2017) Targeting microglial activation states as a therapeutic avenue in parkinson's disease. Front Aging Neurosci 9: 176.
- Boche D, Perry VH, Nicoll JA (2013) Review: activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol 39: 3-18.
- Greter M, Lelios I, Croxford AL (2015) Microglia versus myeloid cell nomenclature during brain inflammation. Front Immunol 6: 249.
- Chiu IM, Morimoto ET, Goodarzi H, Liao JT, O'Keeffe S, et al. (2013) A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep 4: 385-401.
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, et al. (2014) Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat Neurosci 17: 131-143.
- Sasaki A (2017) Microglia and brain macrophages: An update. Neuropathology 37: 452-464.
- Juedes AE, Ruddle NH (2001) Resident and infiltrating central nervous system APCs regulate the emergence and resolution of experimental autoimmune encephalomyelitis. J Immunol 166: 5168-5175.
- Ponomarev ED, Shriver LP, Maresz K, Dittel BN (2005) Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J Neurosci Res 81: 374-389.
- Almolda B, Gonzalez B, Castellano B (2011) Antigen presentation in EAE: role of microglia, macrophages and dendritic cells. Front Biosci (Landmark Ed) 16: 1157-1171.
- Husemann J, Loike JD, Anankov R, Febbraio M, Silverstein SC (2002) Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system. Glia 40: 195-205.
- Peferoen LA, Vogel DY, Ummenthum K, Breur M, Heijnen PD, et al. (2015) Activation status of human microglia is dependent on lesion formation stage and remyelination in multiple sclerosis. J Neuropathol Exp Neurol 74: 48-63.
- Shay T, Kang J (2013) Immunological genome project and systems immunology. Trends Immunol 34: 602-609.
- Heng TS, Painter MW, Immunological Genome Project Consortium (2008) The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol 9: 1091-1094.
- Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, et al. (2014) Colony-stimulating factor 1 receptor signaling is necessary for microglial viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82: 380-397.
- Olmos-Alonso A, Schetters ST, Sri S, Askew K, Mancuso R, et al. (2016) Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer’s-like pathology. Brain 139: 891-907.
- Chitu V, Stanley ER (2017) Regulation of embryonic and postnatal development by the CSF-1 receptor. Curr Top Dev Biol 123: 229-275.
- Kiguchi N, Kobayashi Y, Maeda T, Saika F, Kishioka S (2010) CC-chemokine MIP-1α in the spinal cord contributes to nerve injury-induced neuropathic pain. Neuroscience Letters 484: 17-21.
- Zhu X, Wei D, Chen O, Zhang Z, Xue J, et al. (2016) Upregulation of CCL3/MIP-1alpha regulated by MAPKs and NF-kappaB mediates microglial inflammatory response in LPS-induced brain injury. Acta Neurobiol Exp (Wars) 76: 304-317.
- Passos GF, Figueiredo CP, Prediger RD, Pandolfo P, Duarte FS, et al. (2009) Role of the macrophage inflammatory protein-1alpha/CC chemokine receptor 5 signaling pathway in the neuroinflammatory response and cognitive deficits induced by beta-amyloid peptide. Am J Pathol 175: 1586-1597.
- Zhang, ZJ, Jiang BC, Gao YJ (2017) Chemokines in neuron-glial cell interaction and pathogenesis of neuropathic pain. Cell Mol Life Sci 74: 3275-3291.
- Freria CM, Hall JC, Wei P, Guan Z, McTigue DM, et al. (2017) Deletion of the fractalkine receptor, CX3CR1, improves endogenous repair, axon sprouting, and synaptogenesis after spinal cord injury in mice. J Neurosci 37: 3568-3587.
- Laudati E, Currò D, Navarra P, Lisi L (2017) Blockade of CCR5 receptor prevents M2 microglia phenotype in a microglia-glioma paradigm. Neurochem Int 108: 100-108.
- Maung R, Hoefer MM, Sanchez AB, Sejbuk NE, Medders KE, et al. (2014) CCR5 knockout prevents neuronal injury and behavioral impairment induced in a transgenic mouse model by a CXCR4-using HIV-1 glycoprotein 120. J Immunol 193: 1895-1910.
- Choi DY, Lee MK, Hong JT (2013) Lack of CCR5 modifies glial phenotypes and population of the nigral dopaminergic neurons, but not MPTP-induced dopaminergic neurodegeneration. Neurobiol Dis 49: 159-168.
- Salvi V, Sozio F, Sozzani S, Prete1 AD (2017) Role of atypical chemokine receptors in microglial activation and polarization. Front Aging Neurosci 9: 148.
- Mazzon C, Zanotti L, Wang L, Del Prete A, Fontana E, et al. (2016) CCRL2 regulates M1/M2 polarization during EAE recovery phase. J Leukoc Biol 99: 1027-1033.
- Ravindran C, Cheng YC, Liang SM (2010) CpG-ODNs induces up-regulated expression of chemokine CCL9 in mouse macrophages and microglia. Cell Immunol 260: 113-118.
- Min KJ, Jang JH, Kwon TK (2012) Inhibitory effects of melatonin on the lipopolysaccharide-induced CC chemokine expression in BV2 murine microglial cells are mediated by suppression of Akt-induced NF- κB and STAT/GAS activity. J Pineal Res 52: 296-304.
- Rutar M, Natoli R, Chia RX, Valter K, Proviset JM (2015) Chemokine-mediated inflammation in the degenerating retina is coordinated by Muller cells, activated microglia, and retinal pigment epithelium. J Neuroinflammation 12: 8.
- Kiguchi N, Ding H, Peters CM, Kock ND, Kishioka S, et al. (2017) Altered expression of glial markers, chemokines, and opioid receptors in the spinal cord of type 2 diabetic monkeys. Biochim Biophys Acta 1863: 274-283.
- Kanno M, Suzuki S, Fujiwara T, Yokoyama A, Sakamoto A, et al. (2005) Functional expression of CCL6 by rat microglia: a possible role of CCL6 in cell-cell communication. J Neuroimmunol 167: 72-80.
- Asensio VC, Lassmann S, Pagenstecher A, Steffensen SC, Henriksen SJ, et al. (1999) C10 is a novel chemokine expressed in experimental inflammatory demyelinating disorders that promotes recruitment of macrophages to the central nervous system. Am J Pathol 154: 1181-1191.
- Berjaoui S, Povedano M, Garcia-Esparcia P, Carmona M, Aso E, et al. (2015) Complex Inflammation mRNA-Related Response in ALS Is Region Dependent. Neural Plast 2015: 573784.
- Jemli A, Inoubli O, Trifa F, Mechri A, Zaafrane F, et al. (2017) IFNGR2 genetic polymorphism associated with sex-specific paranoid schizophrenia risk. Nord J Psychiatry 71: 42-47.
- Wilcock DM (2012) Neuroinflammation in the aging down syndrome brain; lessons from Alzheimer’s disease. Curr Gerontol Geriatr Res 2012: 170276.
- Cai J, Zhang W, Yang P, Wang Y, Li M, et al. (2015) Identification of a 6-cytokine prognostic signature in patients with primary glioblastoma harboring M2 microglia/macrophage phenotype relevance. PLoS One 10: 0126022.
- López-González I, Pérez-Mediavilla A, Zamarbide M, Carmona M, Torrejón Escribano B, et al. (2016) Limited unfolded protein response and inflammation in neuroserpinopathy. J Neuropathol Exp Neurol 75: 121-133.
- Godbout JP, Fenn A, Huang Y, Gensel J (2012) Central interleukin-4 infusion after a peripheral lipopolysaccharide injection promotes a neuroprotective CNS environment with increased M2 microglia. Brain, Behavior, and Immunity 26: 29-30.
- Sznejder-Pacholek A, Joniec-Maciejak I, Wawer A, Ciesielska A, Mirowska-Guzel D (2017) The effect of α-synuclein on gliosis and IL-1α, TNFα, IFNγ, TGFβ expression in murine brain. Pharmacol Rep 69: 242-251.
- Rosenkranz K, Tenbusch M, May C, Marcus K, Meier C (2013) Changes in Interleukin-1 alpha serum levels after transplantation of umbilical cord blood cells in a model of perinatal hypoxic-ischemic brain damage. Ann Anat 195: 122-127.
- Luheshi NM, Kovács KJ, Lopez-Castejon G, Brough D, Denes A (2011) Interleukin-1α expression precedes IL-1β after ischemic brain injury and is localised to areas of focal neuronal loss and penumbral tissues. J Neuroinflammation 8: 186.
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, et al. (2016) Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352: 712-716.
- Wyatt SK, Witt T, Barbaro NM, Cohen-Gadol AA, Brewster AL (2017) Enhanced classical complement pathway activation and altered phagocytosis signaling molecules in human epilepsy. Exp Neurol 295: 184-193.
- Watkins LM, Neal JW, Loveless S, Michailidou I, Ramaglia V, et al. (2016) Complement is activated in progressive multiple sclerosis cortical grey matter lesions. J Neuroinflammation 13: 161.
- de Baumont A, Maschietto M, Lima L, Carraro DM, Olivieri EH, et al. (2015) Innate immune response is differentially dysregulated between bipolar disease and schizophrenia. Schizophr Res 161: 215-221.
- Oppenheim JJ (2001) Cytokines: past, present, and future. Int J Hematol 74: 3-8.
- Vitkovic L, Konsman JP, Bockaert J, Dantzer R, Homburger V, et al. (2000) Cytokine signals propagate through the brain. Mol Psychiatry 5: 604-615.
- Deverman BE, Patterson PH (2009) Cytokines and CNS development. Neuron 64: 61-78.
- Becher B, Spath S, Goverman J (2017) Cytokine networks in neuroinflammation. Nat Rev Immunol 17: 49-59.
- Goshen I, Yirmiya R (2009) Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol 30: 30-45.
- Avital A, Goshen I, Kamsler A, Segal M, Iverfeldt K, et al. (2003) Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus 13: 826-834.
- Murray PJ (2006) Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr Opin Pharmaco 6: 379-386.
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A (2001) Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19: 683-765.
- Zlotnik A, Yoshie O (2000) Chemokines: a new classification system and their role in immunity. Immunity 12: 121-127.
- Charo IF, Ransohoff RM (2006) The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 354: 610-621.
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, et al. (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8: 752-758.
- Stence N, Waite M, Dailey ME (2001) Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia 33: 256-266.
- Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308: 1314-1318.
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J (2009) Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci 29: 3974-3980.
- Meeker RB, Williams K, Killebrew DA, Hudson LC (2012) Cell trafficking through the choroid plexus. Cell Adh Migr 6: 390-396.
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, et al. (2011) Synaptic pruning by microglia is necessary for normal brain development. Science 333: 1456-1458.
- Hulshof S, van Haastert ES, Kuipers HF, van den Elsen PJ, De Groot CJ (2003) CX3CL1 and CX3CR1 expression in human brain tissue: noninflammatory control versus multiple sclerosis. J Neuropathol Exp Neurol 62: 899-907.
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, et al. (2006) Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 9: 917-924.
- Marciniak E, Faivre E, Dutar P, Pires CA, Demeyeret D, et al. (2015) The Chemokine MIP-1α/CCL3 impairs mouse hippocampal synaptic transmission, plasticity and memory. Sci Rep 5: 15862.
- Ransohoff RM (2002) Chemokines and chemokine receptors in multiple sclerosis: a few answers and many more questions. In: Ransohoff RM, Suzuki K, Proudfoot AEI, Hickey WF, Harrison JK (eds.). Universes in Delicate Balance: Chemokines and the Nervous System, (edn). Elsevier, Amsterdam, Netherlands.
- Muller DM, Pender MP, Greer JM (2004) Chemokines and chemokine receptors: potential therapeutic targets in multiple sclerosis. Curr Drug Targets Inflamm Allergy 3: 279-290.
- Savarin-Vuaillat C, Ransohoff RM (2007) Chemokines and chemokine receptors in neurological disease: raise, retain, or reduce? Neurotherapeutics 4: 590-601.
- Ubogu EE, Cossoy MB, Ransohoff RM (2006) The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol Sci 27: 48-55.
- Lintner KE, Wu YL, Yang Y, Spencer CH, Hauptmann G, et al. (2016) Early components of the complement classical activation pathway in human systemic autoimmune diseases. Front Immunol 7: 36.
- van Beek J, Elward K, Gasque P (2003) Activation of complement in the central nervous system: roles in neurodegeneration and neuroprotection. Ann N Y Acad Sci 992: 56-71.
- Depboylu C, Schäfer MK, Arias-Carrión O, Oertel WH, Weihe E, et al. (2011) Possible involvement of complement factor C1q in the clearance of extracellular neuromelanin from the substantia nigra in Parkinson disease. J Neuropathol Exp Neurol 70: 125-132.
- Bonifati DM, Kishore U (2007) Role of complement in neurodegeneration and neuroinflammation. Mol Immunol 44: 999-1010.
- Libbey JE, Cusick MF, Doty DJ, Fujinami RS (2017) Complement components are expressed by infiltrating macrophages/activated microglia early following viral infection. Viral Immunol 30: 304-314.
- Fonseca MI, Chu SH, Hernandez MX, Fang MJ, Modarresi L, et al. (2017) Cell-specific deletion of C1qa identifies microglia as the dominant source of C1q in mouse brain. J Neuroinflammation 14: 48.
- Obst J, Simon E, Mancuso R, Gomez-Nicola D (2017) The role of microglia in prion diseases: a paradigm of functional diversity. Front Aging Neurosci 9: 207.
- Ransohoff RM (2016) A polarizing question: do M1 and M2 microglia exist? Nature Neuroscience 19: 987-991.
Citation: von Euler Chelpin M, Arrevillaga-Boni G (2017) Immune Genes Highly Expressed by Microglia: Roles in Physiological and Pathological Conditions in the CNS. J Brain Neursci 1: 001
Copyright: © 2017 Marianne von Euler Chelpin, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

