
Long-Term Outcomes of Treat and Extend Regimen of Anti-Vascular Endothelial Growth Factor in Neovascular Age-Related Macular Degeneration
*Corresponding Author(s):
Manjot K GillDepartment Of Ophthalmology, Northwestern University Feinberg School Of Medicine, 645 North Michigan Avenue, Suite 440, Chicago, IL 60611, United States
Email:mgill@nm.org
Abstract
Keywords
INTRODUCTION
Due to treatment burden, pro re nata (PRN) treatment was studied to examine the effects of monthly follow-up with an individualized retreatment regimen. The PRONTO, IVAN, and CATT trials demonstrated equivalent efficacy between ranibizumab versus bevacizumab; however, there was an overall less favorable outcome in the PRN arms compared to monthly dosing with respect to final visual acuity [6-8].
In 2009, K. Bailey Freund and colleagues were the first to describe the “Treat-And-Extend (TAE)” regimen with treatment of Type 3 CNV lesions in a small cohort over three years [9]. With use of ranibizumab and/or bevacizumab, they showed an overall improvement of vision from 20/80 to 20/40 with an average of 6-7 injections per year. Since then, several other retrospective studies have proposed using a TAE approach as an alternate to monthly or PRN dosing to reduce treatment burden while maintaining or improving visual outcome [10-12].
Despite the multitude of trials demonstrating the safety and efficacy of anti-VEGF drug therapy, there have been limited studies describing the long-term follow-up of anti-VEGF treatment. SEVEN-UP and CATT were among the first studies to describe the long-term outcomes with either monthly or PRN dosing [13,14]. Of the TAE trials, the longest to date was by K. Baily Freund over a 6-year period with 185 unique patients and a retention rate of 62.9% [12]. Their study showed an improvement or maintenance in visual acuity with an average of 8.3 injections per year. They demonstrated that a greater number of injections were an independent predictor of better visual outcome. Other studies have compared TAE to PRN revealing a worse visual outcome with a smaller number of injections with the PRN group [15,16]. Specifically, Calvo et al. showed that over a three-year follow-up period, 42.4% in the TAE dosing group versus 24.1% in the PRN dosing group gained at least three lines of vision. Over the study period, the TAE group was treated with an average of 20.31 injections, while the PRN group was treated with an average of 18.41 [15]. TAE is a practical option to reducing the number of injections and office visits as compared to a monthly and PRN regimen.
Our current study reports on a seven-year follow-up period of the long-term outcomes as measured by visual acuity and OCT imaging of treatment naive NV-AMD patients using a TAE model.
MATERIALS AND METHODS
Subsequent numerical and statistical analyses were performed in Microsoft Excel and Graph Pad Prism. The Student T-test was performed to compare visual acuities at the first and last office visit. Pearson’s correlation coefficient was calculated to test for the linearity of changes in visual acuity over time. Statistical analysis was also performed for visual acuity categories and for the OCT data using contingency tables and p-values were obtained using Fisher’s exact test. Subgroup analysis was conducted on eyes treated with monotherapy to examine the visual acuity and OCT outcomes for each drug.
RESULTS
|
Monotherapy (N=137) |
Multi-drug therapy
|
Total
|
||||
|
Bevacizumab |
Ranibizumab |
Aflibercept |
||||
|
Eye – no (%) |
|
|
|
|
|
|
|
|
OD |
7(37) |
33 (47) |
21 (45) |
48 (55) |
109 (49) |
|
|
OS |
12(63) |
38 (54) |
26 (55) |
39 (45) |
115 (51) |
|
Gender – no (%) |
|
|
|
|
|
|
|
|
F |
16(84) |
49 (69) |
38 (81) |
58 (67) |
161 (72) |
|
|
M |
3(16) |
22 (31) |
9 (19) |
29 (33) |
63 (28) |
|
Race – no (%) |
|
|
|
|
|
|
|
|
Caucasian |
12(63) |
52 (73) |
40 (85) |
72 (83) |
176 (79) |
|
|
African American |
2(11) |
4 (6) |
0 |
5 (6) |
11 (5) |
|
|
Hispanic |
3(16) |
1 (1) |
0 |
2 (2) |
6 (3) |
|
|
Other/Unknown |
2(11) |
14 (20) |
7 (15) |
8 (9) |
31 (14) |
|
Age |
|
|
|
|
|
|
|
|
Mean |
78.1±12.6 |
82.1±6.5 |
83.3±6.4 |
78.2±8.7 |
80.5±8.3 |
|
|
50-69 -no. (%) |
4 (21) |
2 (3) |
2 (4) |
13 (15) |
21 (9) |
|
|
70-89 -no. (%) |
12 (63) |
62 (87) |
40 (85) |
66 (76) |
180 (80) |
|
|
90+ -no. (%) |
3 (16) |
7 (10) |
5 (11) |
8 (9) |
23 (10) |
|
Visual Acuity (Snellen) |
|
|
|
|
|
|
|
|
Mean |
20/94 |
20/124 |
20/91 |
20/89 |
20/100 |
|
|
20/50 or Better - no. (%) |
7 (37) |
19 (27) |
20 (43) |
32 (37) |
78 (35) |
|
|
Between 20/50 and 20/200 - no. (%) |
7 (37) |
29 (41) |
17 (36) |
33 (38) |
86 (38) |
|
|
Worse than 20/200 - no. (%) |
5 (26) |
23 (32) |
10 (21) |
22 (25) |
60 (27) |
|
OCT Findings |
|
|
|
|
|
|
|
|
SRF-no. (%) |
12 (63) |
42 (59) |
30 (64) |
73 (84) |
157 (70) |
|
|
IRF-no. (%) |
8 (42) |
43 (61) |
27 (57) |
28 (32) |
106 (47) |
The average visual acuity at baseline of logMAR 0.698 (20/100 Snellen equivalent) remained stable at the final follow-up visit at logMAR 0.666 (20/93 Snellen) (p=0.52) (Figure 1A). A significant portion of eyes (40%) maintained their visual acuities within two Snellen lines, while 34% of eyes gained more than two lines and 25% lost more than two lines. The percentage of eyes with visual acuities of 20/50 or better increased significantly from 34.8% at baseline to 45.1% by the last follow-up visit (Fischer’s exact test, p=0.037 (Figure 1B). There was no significant difference between baseline IOP (14.8±3.3) and IOP at last follow-up visit (15.2±3.6) (p=0.25).
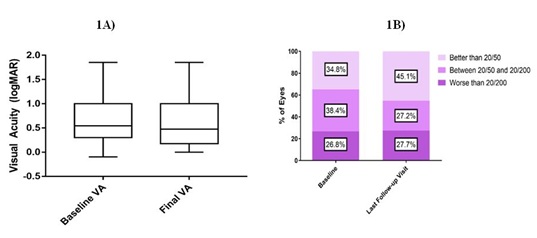
Figure 1A) Shows box-and-whiskers comparisons between baseline and final visual acuities (n = 224). The box represents the interquartile range and the whiskers extend to the minimum and maximum.
Figure 1B) shows the percentage of patients in each visual acuity category by the last follow-up visit compared to baseline.
Visual acuities of patients receiving ongoing injections recorded at 6 months, 1 year and annually thereafter showed an overall steady gain in visual acuity that peaks near the end of the third year, with slight reductions thereafter (Figure 2). The smaller sample size in these groups impedes any individual subgroup analysis of treatment type.
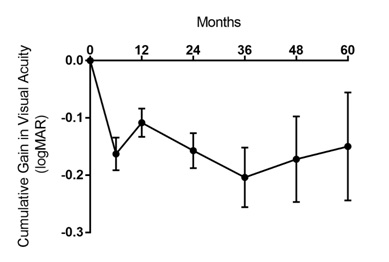 Figure 2:
Figure 2:
Cumulative Gain in logMAR Over Treatment Course (Mean±SE). Visual acuities recorded during patients’ treatment visits were compared with the visual acuity at baseline. Error bars represent Standard Error of the Mean (SEM). Only those patients actively continuing to receive injections were recorded; patients who discontinued injections after a specific time were not included in subsequent time points.
N 224 199 186 137 77 55 40
Mean 0 -0.16 -0.11 -0.16 -0.20 -0.17 -0.15
SE 0 0.03 0.02 0.03 0.05 0.07 0.09
The baseline visual acuity at initial presentation was tested against the change in visual acuity along with demographic factors of sex and age as possible predictors of patient response to treatment. Baseline visual acuities exhibited a weak linear correlation with the cumulative change in visual acuities at all time-points (Pearson’s coefficient r averaged over time points =-0.45, p <0.05). Age was not found to be linearly correlated with the change in visual acuity at last follow-up (Pearson’s r=0.13, p=0.33). Similarly, patients’ sex and race (Caucasian vs. non-Caucasian) also were not correlated with treatment response (t=-1.13, p=0.26 and t=-0.6, p=0.55 respectively).
Over the course of the study, 224 eyes received an average of 20.3±14.7 injections (range 2-95) over an average of 3.4 years of follow-up (range 1.0-7.6), for a total of 4543 injections. 137 (61.1%) patients were treated with a single agent for the duration of their treatment [71 (51.8%) ranibizumab, 47 (34.3%) with aflibercept, and 19 (13.9%) with bevacizumab] while 87 (38.8%) patients were treated with more than one type of agent. For those patients receiving monotherapy, the number of total injections did not differ significantly based on agent used (14.8 for bevacizumab vs. 14.7 for ranibizumab vs. 13.0 for aflibercept, p=0.54) over the course of treatment.
The number of injections that patients received differed over time (Figure 3A). Eyes in the first year of treatment received an average of 8.4 injections and decreased on average by 0.3 injections per year to 5.5 injections by the seventh year (R2 = 0.68). Moreover, eyes that gained more than two lines received significantly more injections with an average total of 24.1±15.3 injections, while eyes that maintained within two lines or lost more than two lines received 18.1±13.3 injections and 18.6±15.2 injections respectively (p<0.05). It was also found that eyes with visual acuities of 20/200 or better at last follow-up tended to receive treatment over a longer period (average 3.1 years vs. 2.2 years, p<0.01) (Figure 3B) and received a greater number of injections (average 23 vs. 14, p<0.001) compared to eyes with visual acuities of 20/200 or worse at last follow-up.
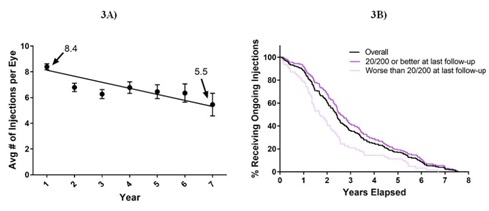 Figure 3: Number of Injections over time and by vision category.
Figure 3: Number of Injections over time and by vision category.Figure 3A) shows the average annual number of injections administered over time. Error bars represent Standard Error of the Mean (SEM).
Figure 3B) shows the percentage of eyes in each visual category continuing to receive ongoing injections over time.
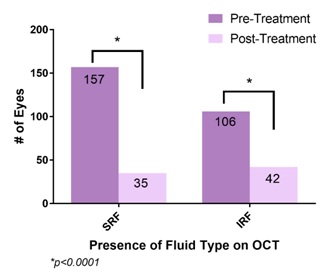 Figure 4: Comparison of OCT finding between baseline and last visit.
Figure 4: Comparison of OCT finding between baseline and last visit.|
# SRF+ Pre-Treatment |
# SRF+ Post-Treatment |
p-value |
# IRF+ Pre-Treatment |
# IRF+ Post-Treatment |
p-value |
|
|
Bevacizumab (N=19) |
14 |
2 |
P<0.01 |
8 |
2 |
P=0.063 |
|
Ranibizumab (N=71) |
42 |
8 |
P<0.001 |
43 |
16 |
P<0.001 |
|
Aflibercept |
30 |
1 |
P<0.001 |
27 |
10 |
P<0.001 |
DISCUSSION
Prior to the development of TAE regimen, long-term outcomes of monthly and PRN anti-VEGF treatments have been described in several other studies, most notably in the SEVEN-UP and 5-year CATT study [13,14]. The SEVEN-UP study reported on ranibizumab-treated patients after an average of 7.3 years from the time of first injection with patients receiving monthly injections in the first two years followed by PRN treatment over the subsequent years. Patients received an average of 6.8 total injections over a mean 3.4-year interval. The subgroup that received more frequent injections yielded a better result in visual acuity gains. In their study, 23% attained a visual acuity of 20/40 or better whereas 37% were 20/200 or worse. There was an overall mean decline of 8.2 letters over the course of follow-up [14].
In the 5-year CATT study, patients were followed an average of 5.5 years from the time of first injection. Ranibizumab or bevacizumab treated patients were stratified into monthly or PRN arms in the first year with the monthly arm stratified again into monthly or PRN treatment in the second year. In the subsequent three years, a variety of treatment drug combinations and regimens were used with patients receiving an average of 15.4 injections over three years. In their study, 49.6% attained visual acuity of 20/40 or better whereas 20% were 20/200 or worse. There was a mean overall decline of 3.3 letters [13].
Since the initial description of the TAE regimen, several studies have reproduced results favouring maintenance or improvement of BCVA similar to monthly dosing while reducing injection frequency and treatment burden. Our study is comparable to others that describe outcomes using a TAE regimen [17-23]. Similar to our study, BCVA in these studies was either maintained or improved throughout treatment with 30-34% of patients on average improving by 2 to 3 lines and 94-97.5% patients losing less than 2-3 lines. The number of injections in the first year averaged a total of 7.6-8.6, which is comparable to our mean of 8.4. Most of these studies, however, only reported on outcomes over a 2-year follow-up while our study looks at outcomes over a longer treatment period. Of note, in our study, the average number of injections decreased to 5.5 during the seventh year while maintaining BCVA.
A study by Mrejen et al., with a longer follow-up period of six years (average 3.5 years), demonstrated similar favorable results [10-12]. In their study, BCVA peaked at 18 months with a steady decline afterwards. On average, patients received 28.5 injections over the study period with 8.3 injections per year and a mean interval of 6.6 weeks between injections. The majority of their patients (64.3%) were treated with a single injection agent of which 59% of them were ranibizumab alone, 4.3% were bevacizumab alone, and 1% was aflibercept alone. Their multivariant analysis shows a greater number of injections as an independent predictor of better visual outcomes. On the other hand, older age of starting injections, hypertension, and anticoagulation were correlated with poorer visual outcomes.
In our current study, we utilize a TAE approach with four physicians in which patients were followed for an average of 3.4 years (range 1.0-7.6) receiving an injection regimen with an average of 20.3±14.7 total injections with 8.4 injections in the first year and 5.5 injections by the seventh year of follow-up. The majority of our patients (61.1%) were also treated with a single anti-VEGF agent for the duration of their treatment of which 51.8% were ranibizumab alone, 34.3% were aflibercept alone, and 13.9% were bevacizumab alone. Having more single agent data analysis allows us to validate similarities of BCVA outcomes regardless of injection drug type. BCVA peaked after 3 years of treatment with a slow decline thereafter. Baseline visual acuity was weakly shown to be the only significant predictor of change in visual acuity. There were no significant differences found between drug type and visual acuity effect, number of injections needed, or OCT outcomes.
Eyes with a final visual acuity of 20/50 or better increased from 34.8% at the beginning of the study to 45.1% at latest follow-up (p=0.037), while eyes with 20/200 or worse remained stable at 26.8% at baseline compared to 27.7% at last follow-up (p=0.92). In contrast, in the 5-year CATT study, eyes with 20/200 or worse increased significantly from 6% at baseline to 20% at the last follow-up [13] even though in our study there were more patients initially with baseline vision of 20/200 or worse. At the end of the SEVEN-UP study, 37% of patients were reported to have visual acuity of 20/200 or worse. Overall 74% of patients in our study maintained or gained at least 2 Snellen lines of visual acuity, compared with the SEVEN-UP trial where only 55% of eyes maintained or improved their vision [14]. Eyes at the end of our study that improved by at least two lines received an average of 24 injections, while all other eyes received an average of 18 injections. Similarly, in the SEVEN-UP study, eyes receiving a greater number of injections (11 vs 6.8) gained 3.9 letters overall and were more likely to show improvement in vision [14].
The better visual outcomes in our population compared to the 5-year CATT study may be explained by the OCT analysis. At the last follow-up visit, 16% of eyes in our study had SRF and 19% of eyes had IRF. Those without SRF or IRF had either a pigment epithelial detachment or were without any fluid. In comparison, at the end of 5 years in the CATT study, 38% had SRF and 61% had IRF [13]. Eyes with residual IRF yield worse visual outcomes compared with eyes with residual SRF or absence of fluid [16]. More frequent treatment may have an impact on amount of fluid on OCT to allow for maintenance or gain in visual acuity. However, other factors such as geographic atrophy also contribute to final visual acuity. Though not studied in our population, the CATT study demonstrated that 24% of eyes with monthly dosing showed geographic atrophy compared to 15% in the PRN group. Similarly, the IVAN trial demonstrated 34% vs 26% showed progressive atrophy in the monthly versus PRN groups respectively [8,13,24].
Our study demonstrates that a TAE model allows for a frequent albeit lower treatment burden as compared to monthly dosing with reduction in fluid on OCT. This may theoretically lower rates of geographic atrophy while maintaining similar gains in visual potential compared with a monthly dosing regimen.
There are several limitations in our study most notably its retrospective nature. With data being compiled via electronic database, records may be incomplete and there may be innate errors in how the data was recorded. Patients began treatment at different times between 2008-2015 and there may be differences both in medical technology and differences in practice patterns amongst providers. There is no monthly regimen treatment arm to compare efficacy to our TAE model. Due to the method of data collection, there were fewer patients with more than 4 to 5 years of treatment available for analysis thereby limiting sample size and comparisons across different anti-VEGF agents. Some patients have undergone cataract surgery during treatment period, which can confound BCVA amongst patients. Furthermore, more in-depth studies are needed to analyze the impact of residual fluid type on visual acuity.
CONCLUSION
DATA AVAILABILITY
CONFLICTS OF INTEREST
FUNDING STATEMENT
ACKNOWLEDGEMENT
REFERENCES
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, et al. (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355: 1419-1431.
- Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, et al. (2009) A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol 148: 43-58.
- Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, et al. (2009) Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology 116: 57-65.
- Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, et al. (2012) Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 119: 2537-2548.
- Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, et al. (2014) Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology 121: 193-201.
- Investigators IS, Chakravarthy U, Harding SP, Rogers CA, Downes SM, et al. (2012) Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 119: 1399-1411.
- Comparison of Age-related Macular Degeneration Treatments Trials Research G, Martin DF, Maguire MG, Fine SL, Ying GS, et al. (2012) Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 119: 1388-1398.
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, et al. (2013) Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet 382: 1258-1267.
- Engelbert M, Zweifel SA, Freund KB (2009) "Treat and extend" dosing of intravitreal antivascular endothelial growth factor therapy for type 3 neovascularization/retinal angiomatous proliferation. Retina 29: 1424-1431.
- Freund KB, Korobelnik JF, Devenyi R, Framme C, Galic J, et al. (2015) Treat-and-Extend Regimens with Anti-Vegf Agents in Retinal Diseases: A Literature Review and Consensus Recommendations. Retina 35: 1489-1506.
- Arnold JJ, Campain A, Barthelmes D, Simpson JM, Guymer RH, et al. (2015) Two-year outcomes of "treat and extend" intravitreal therapy for neovascular age-related macular degeneration. Ophthalmology 122: 1212-1219.
- Mrejen S, Jung JJ, Chen C, Patel SN, Gallego-Pinazo R, et al. (2015) Long-Term Visual Outcomes for a Treat and Extend Anti-Vascular Endothelial Growth Factor Regimen in Eyes with Neovascular Age-Related Macular Degeneration. J Clin Med 4: 1380-1402.
- Comparison of Age-related Macular Degeneration Treatments Trials Research G, Maguire MG, Martin DF, Ying GS, Jaffe GJ, et al. (2016) Five-Year Outcomes with Anti-Vascular Endothelial Growth Factor Treatment of Neovascular Age-Related Macular Degeneration: The Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 123: 1751-1761.
- Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, et al. (2013) Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology 120: 2292-2299.
- Calvo P, Wang Y, Ferreras A, Lam WC, Denevyl R, et al. (2014) Treat and Extend Versus Treat and Observe Regimens in Wet Agerelated Macular Degeneration Patients Treated with Ranibizumab: 3-year Surveillance Period. J Clin Exp Ophthalmol 5: 1-5.
- Oubraham H, Cohen SY, Samimi S, Marotte D, Bouzaher I, et al. (2011) Inject and extend dosing versus dosing as needed: a comparative retrospective study of ranibizumab in exudative age-related macular degeneration. Retina 1: 26-30.
- Holz FG, Tuomi L, Ding B, Hopkins JJ (2015) Development of atrophy in neovascular AMD treated with ranibizumab in the HARBOR study. Invest Ophthalmol Vis Sci 56: 890.
- Gupta OP, Shienbaum G, Patel AH, Fecarotta C, Kaiser RS, et al. (2010) A treat and extend regimen using ranibizumab for neovascular age-related macular degeneration clinical and economic impact. Ophthalmology 117: 2134-2140.
- Rayess N, Houston SK, 3rd, Gupta OP, Ho AC, Regillo CD (2015) Treatment outcomes after 3 years in neovascular age-related macular degeneration using a treat-and-extend regimen. Am J Ophthalmol 159: 3-8.
- Shienbaum G, Gupta OP, Fecarotta C, Patel AH, Kaiser RS, et al. (2012) Bevacizumab for neovascular age-related macular degeneration using a treat-and-extend regimen: clinical and economic impact. Am J Ophthalmol 153: 468-473.
- DeCroos FC, Reed D, Adam MK, Salz D, Gupta OP, et al. (2017) Treat-and-Extend Therapy Using Aflibercept for Neovascular Age-related Macular Degeneration: A Prospective Clinical Trial. Am J Ophthalmol 180: 142-150.
- Wykoff CC, Ou WC, Brown DM, Croft DE, Wang R, et al. (2017) Randomized Trial of Treat-and-Extend versus Monthly Dosing for Neovascular Age-Related Macular Degeneration: 2-Year Results of the TREX-AMD Study. Ophthalmology Retina 1: 314-321.
- Jaffe GJ, Martin DF, Toth CA, Daniel E, Maguire MG, et al. (2013) Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials. Ophthalmology 120: 1860-1870.
- Abedi F, Wickremasinghe S, Islam AF, Inglis KM, Guymer RH (2014) Anti-VEGF treatment in neovascular age-related macular degeneration: a treat-and-extend protocol over 2 years. Retina 34: 1531-1538.
Citation: Lee A, Garg PG, Fawzi AA, Lyon AT, Mirza R, et al. (2019) Long-Term Outcomes of Treat and Extend Regimen of Anti-Vascular Endothelial Growth Factor in Neovascular Age-Related Macular Degeneration. J Ophthalmic Clin Res 6: 056.
Copyright: © 2019 Andy Lee, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

