
Journal of Ophthalmology & Clinical Research Category: Clinical
Type: Research Article
Ozurdex Outcomes in Retinal Vein Occlusion with Macular Edema
*Corresponding Author(s):
Valerie JuniatSussex Eye Hospital, Eastern Road, Brighton, BN2 5BF, United Kingdom
Tel:+44 01273606126,
Email:vjuniat@doctors.org.uk
Received Date: Feb 14, 2015
Accepted Date: Feb 20, 2015
Published Date: Mar 06, 2015
Abstract
IntroductionOzurdex is a biodegradable intravitreal steroid implant that is currently approved by the National Institute for Health and Care Excellence for treatment of patients with macular edema due to retinal vein occlusions in the United Kingdom.
PurposeThis retrospective study aimed to evaluate the visual outcomes, anatomical outcomes, and complications in patients treated with Ozurdex for macular edema secondary to retinal vein occlusions.
MethodsData was collected using Medisoft and Topcon Optical Coherence Tomography (OCT) imaging. Data was analysed using Microsoft Excel and statistical analysis was carried out using MedCalc software.
Results181 Ozurdex implants were carried out over 15 months between 2012 and 2013. 6% were lost to follow up. 107 procedures were included in the study. Prior to first Ozurdex implantation, 26 patients with BRVO had previous grid laser treatment, 3 CRVO patients had Pan-Retinal Photocoagulation (PRP), 11 patients had anti-VEGF treatment, and 6 patients had intravitreal triamcinolone injections. The average time interval between pre-treatment baseline visual acuity and post-treatment final visual acuity was 6 months (median 2 to 19 months). Mean difference in visual acuity post-treatment was -0.08 (LogMAR). OCT analysis showed average central retinal thickness reduction of 149µm post-treatment. Complications included raised Intraocular Pressure (IOP) requiring IOP-lowering treatment (15.0%), of which 3 patients had IOP >35mmHg (2.8%); cataract formation requiring extraction (1.9%); and conversion into ischemic vein occlusion (2.8%, or 2 patients with central retinal vein occlusion and one with branch retinal vein occlusion).
RecommendationsThis study demonstrated that Ozurdex implantations achieved anatomical improvement in patients with macular edema secondary to retinal vein occlusions that did not correlate with visual improvement. The study recommends a dedicated retinal vein occlusion service to ensure patients are reviewed in clinic at appropriate time intervals to achieve better outcomes. Alternative treatment modalities such as laser treatment and anti vascular endothelial growth factor injections should be available for patients who do not achieve visual improvement with Ozurdex, and vice versa.
PurposeThis retrospective study aimed to evaluate the visual outcomes, anatomical outcomes, and complications in patients treated with Ozurdex for macular edema secondary to retinal vein occlusions.
MethodsData was collected using Medisoft and Topcon Optical Coherence Tomography (OCT) imaging. Data was analysed using Microsoft Excel and statistical analysis was carried out using MedCalc software.
Results181 Ozurdex implants were carried out over 15 months between 2012 and 2013. 6% were lost to follow up. 107 procedures were included in the study. Prior to first Ozurdex implantation, 26 patients with BRVO had previous grid laser treatment, 3 CRVO patients had Pan-Retinal Photocoagulation (PRP), 11 patients had anti-VEGF treatment, and 6 patients had intravitreal triamcinolone injections. The average time interval between pre-treatment baseline visual acuity and post-treatment final visual acuity was 6 months (median 2 to 19 months). Mean difference in visual acuity post-treatment was -0.08 (LogMAR). OCT analysis showed average central retinal thickness reduction of 149µm post-treatment. Complications included raised Intraocular Pressure (IOP) requiring IOP-lowering treatment (15.0%), of which 3 patients had IOP >35mmHg (2.8%); cataract formation requiring extraction (1.9%); and conversion into ischemic vein occlusion (2.8%, or 2 patients with central retinal vein occlusion and one with branch retinal vein occlusion).
RecommendationsThis study demonstrated that Ozurdex implantations achieved anatomical improvement in patients with macular edema secondary to retinal vein occlusions that did not correlate with visual improvement. The study recommends a dedicated retinal vein occlusion service to ensure patients are reviewed in clinic at appropriate time intervals to achieve better outcomes. Alternative treatment modalities such as laser treatment and anti vascular endothelial growth factor injections should be available for patients who do not achieve visual improvement with Ozurdex, and vice versa.
Keywords
Macular Edema; Ozurdex; Retinal vein occlusion
INTRODUCTION
Retinal Vein Occlusion (RVO) is a vascular disorder that occurs following an obstruction in the retinal venous system, and may involve the central, hemi-central or branch retinal vein [1]. RVOs are the second commonest cause of reduced vision due to retinal vascular disease after diabetic retinopathy, with Branch Retinal Vein Occlusions (BRVOs) occurring 2-3 times more commonly than Central Retinal Vein Occlusions (CRVOs) [2]. The incidence of RVOs rises with increasing age [3]. Patients commonly present with blurred vision, but may be asymptomatic with RVO found during routine examination.
The most common sight-threatening complications are Macular Edema (ME) and retinal ischemia. ME can resolve without treatment in about one third of patients with non-ischemic CRVO and in 18-41% of BRVO patients who have ME at baseline examination [4,5]. Laser photocoagulation was the standard therapy for patients with ME secondary to BRVO, but it was not found to have benefit in patients with ME secondary to CRVO [6,7]. More recently, studies have shown benefit of steroid implantation and anti-Vascular Endothelial Growth Factor (anti-VEGF) therapies for the management of patients with ME secondary to RVO [8-10].
Ozurdex is a biodegradable intravitreal dexamethasone implant that received National Institute for Health and Clinical Excellence (NICE) approval for treatment of patients with ME due to RVO in 2011 [11]. More recently, NICE has also approved the use of ranibizumab (Lucentis), an intravitreal anti-VEGF therapy, as treatment for the same condition [11-13]. We describe a retrospective case series to determine the visual outcomes, anatomical outcomes and complications for RVO patients treated with Ozurdex implants.
The most common sight-threatening complications are Macular Edema (ME) and retinal ischemia. ME can resolve without treatment in about one third of patients with non-ischemic CRVO and in 18-41% of BRVO patients who have ME at baseline examination [4,5]. Laser photocoagulation was the standard therapy for patients with ME secondary to BRVO, but it was not found to have benefit in patients with ME secondary to CRVO [6,7]. More recently, studies have shown benefit of steroid implantation and anti-Vascular Endothelial Growth Factor (anti-VEGF) therapies for the management of patients with ME secondary to RVO [8-10].
Ozurdex is a biodegradable intravitreal dexamethasone implant that received National Institute for Health and Clinical Excellence (NICE) approval for treatment of patients with ME due to RVO in 2011 [11]. More recently, NICE has also approved the use of ranibizumab (Lucentis), an intravitreal anti-VEGF therapy, as treatment for the same condition [11-13]. We describe a retrospective case series to determine the visual outcomes, anatomical outcomes and complications for RVO patients treated with Ozurdex implants.
MATERIAL AND METHODS
Ozurdex (0.7mg dexamethasone) implants carried out in Kent & Canterbury hospital over a 15-month period were analysed. Our follow up schedule included a 2-week post-procedure Intraocular Pressure (IOP) check, followed by 8-weekly IOP and Optical Coherence Tomography (OCT) checks. Patients are considered for a repeat injection if required after 4-6 months from the initial treatment. Indications for repeat injection include worsening Visual Acuity (VA) and/or worsening macular edema with increased Central Retinal Thickness (CRT) on OCT.
Patient data were retrospectively obtained from Medisoft™ and from paper records. Categories of information collected include patient demographics, date of diagnosis, history of previous treatments, date of visits, visual acuity as measured using the LogMAR scale pre-treatment and at each post-treatment follow up visit, CRT measurements pre-treatment and at each post-treatment follow up visit, and any complications consequent to treatment. Final VA in LogMAR was defined as Best-Corrected Visual Acuity (BCVA) at the 6-month post-treatment visit or VA at visit where decision was taken to retreat with Ozurdex implantation. OCT macular imaging was captured and analysed using Topcon software. Results were extracted and analysed using Microsoft Excel. Statistical comparison was carried out using Medcalc™ software.
Patient data were retrospectively obtained from Medisoft™ and from paper records. Categories of information collected include patient demographics, date of diagnosis, history of previous treatments, date of visits, visual acuity as measured using the LogMAR scale pre-treatment and at each post-treatment follow up visit, CRT measurements pre-treatment and at each post-treatment follow up visit, and any complications consequent to treatment. Final VA in LogMAR was defined as Best-Corrected Visual Acuity (BCVA) at the 6-month post-treatment visit or VA at visit where decision was taken to retreat with Ozurdex implantation. OCT macular imaging was captured and analysed using Topcon software. Results were extracted and analysed using Microsoft Excel. Statistical comparison was carried out using Medcalc™ software.
RESULTS
181 Ozurdex implantations were carried out during this period. 74 procedures were excluded from our case series, including 11 cases that were lost to follow up (6%) (Figure 1).
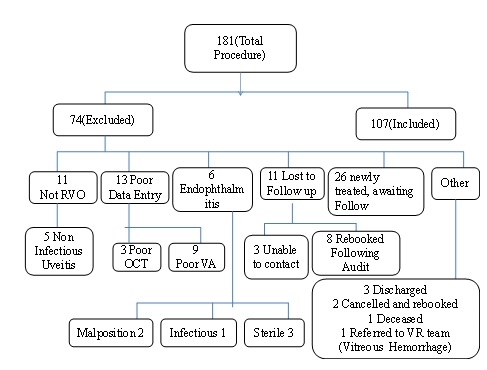
Figure 1: Total number of Ozurdex injections carried out during study period.
Of the 107 procedures included, 46 were carried out in patients with ME secondary to BRVO who were not amenable for laser treatment, and 61 in ME secondary to CRVO. 48% of the BRVO eyes had severe haemorrhage at baseline, defined as 4 or more optic disc diameters of haemorrhage. The mean age of treatment was 72.6 years (37-94 years). 49 procedures were carried out in men and 58 in women. During this 15-month period, 60 patients had one Ozurdex implantation, 22 patients had two implantations and 1 patient had three implantations. Prior to first Ozurdex implantation, 26 patients with BRVO had previous grid laser treatment, 3 CRVO patients had Pan-Retinal Photocoagulation (PRP), 11 patients had anti-VEGF treatment, and 6 patients had intravitreal triamcinolone injections.
VISUAL OUTCOMES
The mean baseline VA pre-treatment was 1.15 LogMAR (0.12-2.7 LogMAR, Standard Deviation [SD] 0.71) and post-treatment was 1.23 LogMAR (-0.1-3.0 LogMAR, SD 0.80). The mean difference in VA following treatment was an improvement of 0.08 LogMAR (-2.84-1.2 LogMAR, SD 0.55) or 4-letter gain. Change in LogMAR VA does not show any correlation with baseline VA.
The mean interval between diagnosis and first treatment was 394 days. 30 patients were treated within 180 days of diagnosis and showed a mean loss of VA of 0.24 LogMAR (or 12-letter loss). 26 patients were treated 180-365 days of diagnosis and showed a mean gain of VA of 0.10 LogMAR (or 5-letter gain). 40 patients were treated 1-2 years from diagnosis and showed a mean loss of VA of 0.08 LogMAR (or 4-letter loss). 9 patients were treated more than 2 years from diagnosis and showed no change to VA with 0.0 LogMAR.
The mean interval between baseline VA pre-treatment and final VA following treatment was 6.3 months, or 189.9 days (61-228 days, SD 77.0). Of note, 15 procedures were followed up within 120 days, 38 within 120 to 180 days, and 54 at more than 180 days.
Change in LogMAR VA does not show any correlation with time interval of follow up post injection up to 180 days. There is worsening LogMAR VA 180 days after treatment.
The mean interval between diagnosis and first treatment was 394 days. 30 patients were treated within 180 days of diagnosis and showed a mean loss of VA of 0.24 LogMAR (or 12-letter loss). 26 patients were treated 180-365 days of diagnosis and showed a mean gain of VA of 0.10 LogMAR (or 5-letter gain). 40 patients were treated 1-2 years from diagnosis and showed a mean loss of VA of 0.08 LogMAR (or 4-letter loss). 9 patients were treated more than 2 years from diagnosis and showed no change to VA with 0.0 LogMAR.
The mean interval between baseline VA pre-treatment and final VA following treatment was 6.3 months, or 189.9 days (61-228 days, SD 77.0). Of note, 15 procedures were followed up within 120 days, 38 within 120 to 180 days, and 54 at more than 180 days.
Change in LogMAR VA does not show any correlation with time interval of follow up post injection up to 180 days. There is worsening LogMAR VA 180 days after treatment.
ANATOMICAL OUTCOMES
The mean CRT pre-treatment was 534.0µm (290-1493µm, SD 159.4). The mean CRT post-treatment was 372.7µm (186-1164µm, SD 127.0). There is an average reduction of CRT of 149µm (-1273 to +348µm, SD 189.2) following treatment. There is no association between change in VA and change in CRT following treatment, regardless of the baseline VA (correlation coefficient r 0.1413, p=0.1465, 95% CI -0.04987 to 0.3225) (Figure 2).
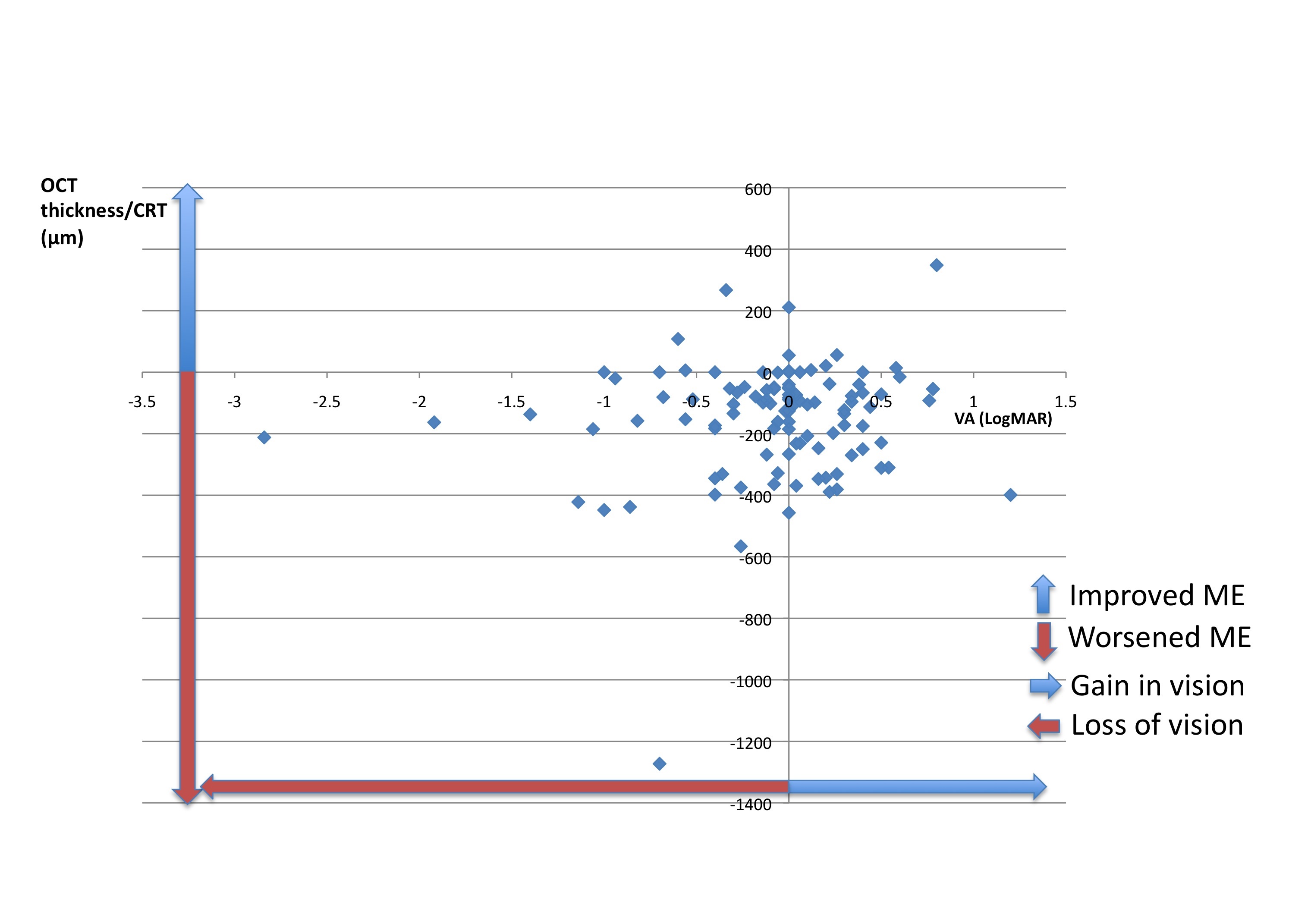
Figure 2: Association between change in VA (LogMAR) and change in CRT (μm). There is no association between change in VA and change in CRT following treatment.
Patients who have had more than one Ozurdex injection showed an average CRT reduction of 185µm post treatment. There is no association between change in VA and change in CRT following second treatment (correlation coefficient r 0.4099, p=0.058, 95% CI -0.01418 to 0.7090).
Of patients with baseline BCVA <6/24 Snellen, there was an average loss of VA of 0.16 LogMAR (8 letters), with 44% showing stable or improved VA and CRT post treatment. Of patients with baseline BCVA 6/24-6/60 Snellen, there was an average loss of VA of 0.14 LogMAR (7 letters), with 37% showing stable or improved VA and CRT post treatment. Of patients with baseline BCVA >6/60, there was an average of no change in VA post treatment (0.0 LogMAR), with 76% showing stable or improved VA and CRT post treatment (Figure 3).

Figure 3: Association between change in VA (LogMAR) and change in CRT (μm), classified according to baseline BCVA pre-treatment. There is no association between change in VA and change in CRT following treatment, regardless of the baseline VA.
Complications
The most common post-operative complication was raised Intraocular Pressure (IOP) requiring IOP-lowering treatment (15.0%, 16/107), of which 3 patients had IOP >35mmHg (2.8%, 3/107). All patients responded to topical anti-glaucoma medication and no glaucoma surgery was required. Other complications included cataract formation requiring extraction (1.9%, 2/107), and ischemic conversion (2.8%, 3/107 - two patients with a baseline diagnosis of CRVO and one with BRVO).
Co-pathology
12.1% (13/107) patients had co-existing retinal pathologies. 5 had Epiretinal Membranes (ERM) and 8 had macular ischemia secondary to vein occlusion on Fundus Fluorescein Angiogram (FFA). We removed these 13 patients from our 107 cases for further analysis. The mean difference in VA following treatment was an improvement of 0.08 LogMAR (-2.84-1.2 LogMAR, SD 0.57) and there was an average reduction of CRT of 147µm (-1273 to +348µm, SD 195.0) following treatment.
DISCUSSION
NICE guidelines recommend using dexamethasone intravitreal implant (Ozurdex) as an option for the treatment of ME following CRVO, or for the treatment of ME following BRVO when treatment with laser photocoagulation has not been beneficial or where treatment with laser photocoagulation is not considered suitable due to the extent of macular haemorrhage [11]. There are no limitations based on baseline VA, CRT or duration of condition. Our case series aimed to determine the visual outcomes, anatomical outcomes and complications seen in patients treated with Ozurdex implantation for ME secondary to RVO.
VISUAL OUTCOME
The mean interval between baselines VA pre-treatment and final VA following treatment was 6.3 months (median 2 to 19 months). However in our analysis this does not seem to have any correlation with change in VA.
We found that VA remained stable overall following treatment, with a mean improvement of 0.08 LogMAR (4-letter gain), with the largest improvement seen in the subgroup of patients who were treated within 180-365 days of diagnosis. Our overall improvement in VA is comparable with other studies [14]. However, it is a smaller gain in VA post treatment compared with findings from the GENEVA trial [9]. This could be because 45.8% (49/107) patients in our study had ME for more than 12 months before they received their first Ozurdex implantation. Our findings are consistent with other studies suggesting that longer duration of ME due to RVO before first treatment with Ozurdex is associated with a significantly lower likelihood of visual or anatomical improvements 6 or 12 months after treatment [9,15]. Treatment of longstanding ME is less likely to lead to improvement of VA due to damage to the underlying neurosensory retinal layer, and this has been shown in subgroup analyses in the BVOS, SCORE-BRVO, and BRAVO clinical trials, all suggesting that a shorter disease duration may be more likely to have clinically significant improvements in vision in response to treatment than did patients with longer disease duration [6,15-17].
44% of patients with baseline BCVA <6/24 Snellen, 37% of patients with baseline BCVA 6/24-6/60 Snellen, and 76% of patients with baseline BCVA >6/60 Snellen showed stable or improved VA and CRT post operatively. Our results showed that a larger proportion of patients with baseline BCVA >6/60 Snellen appeared to show stable or improved VA compared with patients within other VA subgroups. Although it may appear that the treatment results are not as good for patients with good baseline VA, there is a greater range of possible outcomes for these patients as their vision may remain stable, improve or worsen. Patients with poor baseline VA do not generally see a deterioration of their vision as a result of this treatment as their vision is already at a point where the treatment cannot cause significant deterioration. As a result the numbers for patients with poor vision only reflect cases where vision improves or vision remains stable, and this makes the numbers for those patients appear artificially positive.
We found that VA remained stable overall following treatment, with a mean improvement of 0.08 LogMAR (4-letter gain), with the largest improvement seen in the subgroup of patients who were treated within 180-365 days of diagnosis. Our overall improvement in VA is comparable with other studies [14]. However, it is a smaller gain in VA post treatment compared with findings from the GENEVA trial [9]. This could be because 45.8% (49/107) patients in our study had ME for more than 12 months before they received their first Ozurdex implantation. Our findings are consistent with other studies suggesting that longer duration of ME due to RVO before first treatment with Ozurdex is associated with a significantly lower likelihood of visual or anatomical improvements 6 or 12 months after treatment [9,15]. Treatment of longstanding ME is less likely to lead to improvement of VA due to damage to the underlying neurosensory retinal layer, and this has been shown in subgroup analyses in the BVOS, SCORE-BRVO, and BRAVO clinical trials, all suggesting that a shorter disease duration may be more likely to have clinically significant improvements in vision in response to treatment than did patients with longer disease duration [6,15-17].
44% of patients with baseline BCVA <6/24 Snellen, 37% of patients with baseline BCVA 6/24-6/60 Snellen, and 76% of patients with baseline BCVA >6/60 Snellen showed stable or improved VA and CRT post operatively. Our results showed that a larger proportion of patients with baseline BCVA >6/60 Snellen appeared to show stable or improved VA compared with patients within other VA subgroups. Although it may appear that the treatment results are not as good for patients with good baseline VA, there is a greater range of possible outcomes for these patients as their vision may remain stable, improve or worsen. Patients with poor baseline VA do not generally see a deterioration of their vision as a result of this treatment as their vision is already at a point where the treatment cannot cause significant deterioration. As a result the numbers for patients with poor vision only reflect cases where vision improves or vision remains stable, and this makes the numbers for those patients appear artificially positive.
ANATOMICAL OUTCOME
Our patients showed an overall anatomical improvement in CRT following Ozurdex implantation, with an average reduction of 149µm following the first injection and a reduction of 185µm following the second injection. This compares favourably with results from the GENEVA trial, which showed a mean reduction of 119µm at 180 days following treatment [9], as well as other studies [14,18,19]. However there was no association between change in VA and change in CRT following treatment in our study, regardless of the baseline VA. As improvement in CRT does not necessarily reflect visual improvement, we surmise that resolution of ME on OCT scanning may leave residual macular atrophy or scarring with consequently little improvement in VA.
Complications
The most common post-operative complication seen was raised intraocular pressure which responded to topical antiglaucoma medication alone (15.0%), of which 2.8% had IOP >35mmHg. These rates are comparable to the GENEVA trial, where they reported around 16% of their study patients had IOP >25mmHg and 3.2% had IOP>35mmHg at its peak [9]. Patients with endophthalmitis were excluded from our case series, but our endophthalmitis rate was 3.3% (6/181), higher than that seen in the GENEVA study (0%). 2 patients had malpositioned implants, which occurred within the first two months of our institution. Similar cases have been reported elsewhere in the literature [20,21]. Following a review of our local procedure, there was a change in injection technique and we have not experienced any further episodes.
Co-pathology
48% of BRVO patients had severe haemorrhage in treated eyes, which may be the cause of poor outcome. Additionally, the existence of other retinal pathologies was noted in 12% of patients. However there was minimal difference to our visual and anatomical results following the exclusion of this group of patients from our overall results.
Limitations
There are currently no limitations according to NICE guidelines on which patients can receive Ozurdex for ME secondary to RVO. Patients with longstanding ME were therefore offered Ozurdex implantation at our institution. The resultant visual outcome could be affected by this factor, as discussed previously.
Where possible, a Fundus Fluorescein Angiogram (FFA) was carried out for all patients to evaluate the presence of macular ischemia prior to initiating treatment. However, this was not always possible due to time and resource constraints. It is therefore possible that some patients within our study had macular ischemia, which would further adversely affect visual outcome. OCT software automatically calculates CRT from scans. There may be inaccuracies if there is gross ME or underlying haemorrhage.
Finally, our local follow up protocol allowed for patients to be reviewed at or near their follow up time, as opposed to strictly adhering to an 8-weekly schedule. It is therefore not possible to directly compare the results at 3,6 and 12 months, were applicable. It could be argued that this reflects the real-world scenario, where patients are reviewed in busy and pressurised retinal clinics. Additionally, 6% of patients (11/181) were lost to follow up. These patients were contacted following the results of the study to arrange a clinic appointment. These points highlight the need to track patients following treatment, and the importance of having a dedicated RVO service with an RVO coordinator.
Where possible, a Fundus Fluorescein Angiogram (FFA) was carried out for all patients to evaluate the presence of macular ischemia prior to initiating treatment. However, this was not always possible due to time and resource constraints. It is therefore possible that some patients within our study had macular ischemia, which would further adversely affect visual outcome. OCT software automatically calculates CRT from scans. There may be inaccuracies if there is gross ME or underlying haemorrhage.
Finally, our local follow up protocol allowed for patients to be reviewed at or near their follow up time, as opposed to strictly adhering to an 8-weekly schedule. It is therefore not possible to directly compare the results at 3,6 and 12 months, were applicable. It could be argued that this reflects the real-world scenario, where patients are reviewed in busy and pressurised retinal clinics. Additionally, 6% of patients (11/181) were lost to follow up. These patients were contacted following the results of the study to arrange a clinic appointment. These points highlight the need to track patients following treatment, and the importance of having a dedicated RVO service with an RVO coordinator.
CONCLUSION
What was known before?
- Retinal vein occlusions are the second commonest cause of reduced vision due to retinal vascular disease after diabetic retinopathy, with branch retinal vein occlusions occurring 2-3 times more commonly than central retinal vein occlusions.
- The most common sight-threatening complications are macular edema and retinal ischemia.
- Ozurdex is a biodegradable intravitreal dexamethasone implant that received National Institute for Health and Clinical Excellence (NICE) approval for treatment of patients with macular edema due to retinal vein occlusions in 2011.
What this study adds
- Patients in our case series showed anatomical improvement and an average of 4-letter visual gain.
- Poor baseline visual acuity, duration of macular edema and existing co- pathology could have contributed towards lack of visual improvement.
- Improvement in patient selection and early initiation of treatment may lead to more favourable visual outcome.
- Combination treatment with laser therapy and/or anti-VEGF treatment with Ozurdex implantations may improve outcomes.
Patients in our case series showed anatomical improvement and an average of 4-letter visual gain following Ozurdex implantation for ME secondary to RVOs. This is a smaller visual improvement compared with results from the GENEVA study. Improvement in patient selection may lead to more favourable visual outcome. We recommend early initiation of treatment, which includes educating opticians for early referral. Our study also highlighted a lack of rigorous measures for reviewing patients post treatment. We recommend a dedicated RVO service to ensure patients are reviewed in clinic at appropriate time intervals to achieve better outcomes. Alternative treatment modalities such as laser treatment and anti-VEGF injections should be available for patients who do not achieve visual improvement with Ozurdex, and vice versa. Ultra-wide field imaging with angiography can delineate areas of non-perfusion that would lead to neovascularization, and targeted retinal laser therapy as a combination treatment may help demonstrate better outcomes. We aim to carry out a further study to determine visual outcomes for patients treated with anti-VEGF injections for this condition. Non-responders to Ozurdex may have to be given a guarded prognosis to visual outcome if anti-VEGF treatment is being considered as a second line option
REFERENCES
- The Royal College of Ophthalmologists (2010) Interim Guidelines for Management of Retinal Vein Occlusion, London, UK. Pg: 1-66.
- Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, et al. (2010) The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology 117: 313-319.
- Mitchell P, Smith W, Chang A (1996) Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol 114: 1243-1247.
- McIntosh RL, Rogers SL, Lim L, Cheung N, Wang JJ, et al. (2010) Natural history of central retinal vein occlusion: an evidence-based systematic review. Ophthalmology 117: 1113-1123.
- Rogers SL, McIntosh RL, Lim L, Mitchell P, Cheung N, et al. (2010) Natural history of branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology 117: 1094-1101.
- [No authors listed] (1984) Argon laser photocoagulation for macular edema in branch vein occlusion. The Branch Vein Occlusion Study Group. Am J Ophthalmol 98: 271-282.
- [No authors listed] (1995) Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. The Central Vein Occlusion Study Group M report. Ophthalmology 102: 1425-1433.
- Brown DM, Campochiaro PA, Singh RP, Li Z, Gray S, et al (2010) Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 117: 1124-1133.
- Haller JA, Bandello F, Belfort R Jr, Blumenkranz MS, Gillies M, et al (2010) Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology 117: 1134-1146.
- Korobelnik JF, Holz FG, Roider J, Ogura Y, Simader C, et al. (2014) Intravitreal Aflibercept Injection for Macular Edema Resulting from Central Retinal Vein Occlusion: One-Year Results of the Phase 3 GALILEO Study. Ophthalmology 121: 202-208.
- NICE guidelines (2011) Dexamethasone intravitreal implant for the treatment of macular edema secondary to retinal vein occlusion.
- NICE guidelines (2013) Ranibizumab for treating visual impairment caused by macular edema secondary to retinal vein occlusion.
- Ford JA, Clar C, Lois N, Barton S, Thomas S, et al. (2014) Treatments for macular oedema following central retinal vein occlusion: systematic review. BMJ Open 4: 004120.
- Moisseiev E, Goldstein M, Waisbourd M, Barak A, Loewenstein A (2013) Long-term evaluation of patients treated with dexamethasone intravitreal implant for macular edema due to retinal vein occlusion. Eye (Lond) 27: 65-71.
- Yeh WS, Haller JA, Lanzetta P, Kuppermann BD, Wong TY, et al. (2012) Effect of the duration of macular edema on clinical outcomes in retinal vein occlusion treated with dexamethasone intravitreal implant. Ophthalmology 119: 1190-1198.
- Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, et al. (2010) Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 117: 1102-1112.
- Scott IU, Ip MS, VanVeldhuisen PC, Oden NL, Blodi BA, et al. (2009) A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular Edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch Ophthalmol 127: 1115-1128.
- Querques G, Lattanzio R, Querques L, Triolo G, Cascavilla ML, et al. (2014) Impact of intravitreal dexamethasone implant (Ozurdex) on macular morphology and function. Retina 34: 330-341.
- Bezatis A, Spital G, Höhn F, Maier M, Clemens CR, et al. (2013) Functional and anatomical results after a single intravitreal Ozurdex injection in retinal vein occlusion: a 6-month follow-up -- the SOLO study. Acta Ophthalmol 91: 340-347.
- Coca-Robinot J, Casco-Silva B, Armadá-Maresca F, García-Martínez J (2014) Accidental injections of dexamethasone intravitreal implant (Ozurdex) into the crystalline lens. Eur J Ophthalmol 24: 633-636.
- Berarducci A, Sian IS, Ling R (2014) Inadvertent dexamethasone implant injection into the lens body management. Eur J Ophthalmol 24: 620-622.
Citation: Juniat V, Tsolakou E, Patel N (2015) Ozurdex Outcomes in Retinal Vein Occlusion with Macular Edema. J Ophthalmic Clin Res 2: 008.
Copyright: © 2015 Valerie Juniat, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2026, Copyrights Herald Scholarly Open Access. All Rights Reserved!

