
Personalized Glaucoma Medication Compounding Reduces Intraocular Pressure and May Increase Adherence and Affordability
*Corresponding Author(s):
Daniel LarocheAdvanced Eye Care Of New York, 49 West 127th Street, NY, NY 10027, United States
Tel:+1 2126630473,
Fax:+1 9174931010
Email:dlarochemd@aol.com
Abstract
Glaucoma treatment relies on Intraocular Pressure (IOP) reduction through surgical or pharmacological means. Failure or refusal of surgery results in the use of multiple medications that can be costly and burdensome. We examined the effects of in-office compounding of patients’ prescribed glaucoma medications on IOP reduction and adherence. We report results from four glaucoma patients on multiple topical medications who refused or failed interventional surgery and opted for in-office medication compounding (n=4). Topical medications with preservatives were compounded into one 10ml-bottle under sterile conditions. Patients used one drop of the compounded solution twice daily. Patients demonstrated significantly reduced IOP and self-reported better compliance after 3 months of use. They also reported that compounded medication was less expensive, more effective, and easier to implement. Personalized in-office medication compounding is a promising, low-cost, patient-centered technique that reduces IOP and may improve patient adherence, and further studies are needed to assess its feasibility.
Keywords
Adherence; Compounding; Glaucoma; Therapeutics
Introduction
Glaucoma is an optic neuropathy in which damage to the retinal ganglion cells and their axons can result in blindness. It is the second leading cause of blindness worldwide [1]. The exact etiology of the degenerative damage to the optic nerve seen in glaucoma is multifactorial, and risk factors include older age, increased cup-to-disc ratio of the optic nerve, a thin central cornea, family history, and increased Intraocular Pressure (IOP) [2]. IOP is the only known modifiable risk factor; the greater the IOP, the faster damage progresses. Glaucoma treatment is therefore focused on reducing IOP through surgery, laser, or pharmacological therapy, and medications are generally the first step. While the initial approach to adult glaucoma is often surgical and often involves early cataract extraction to lower IOP and accompanying angle surgery to restore outflow via Schlemm’s canal, some patients refuse or fail surgery and require multiple medications for disease management. Often, more than one medication is needed to meet and maintain the target IOP, and effectiveness of treatment is limited by treatment adherence [3]. Medications also have the active ingredients and preservatives that can lead to ocular erythema and conjunctivitis, further reducing compliance [4]. Compounding multiple topical agents into a single solution has several benefits when compared with monotherapy: reduced burden of therapy, cost savings, reduced exposure to preservatives, and easier use. However, the doses and concentrations in commercially available combinations are fixed, and this does not allow for dosing flexibility for each component. Moreover, if more than 2 medications are needed, the patient is again forced to keep track of multiple medications, potentially more frequent doses, and additional cost.
Though fixed combination therapies have been investigated, no study to date has reported the efficacy of personalized combination of all prescribed topical agents into one bottle. Many compounding pharmacies do not take most managed care insurances, making their products inaccessible to many patients due to additional costs. Here we report the results of twice-daily administration of a sterile compounded solution of all of an individual patient’s glaucoma medications in four patients with glaucoma.
Case Report
Case 1
A 74-year-old male with a history of glaucoma, cataract, and hypertension presented for a follow-up visit. The patient had an extensive history of ocular surgery including trabeculoplasty in both eyes, trabeculectomy in the left eye (OS), trabeculectomy in the right eye (OD) twice, and Ahmed valve implant OD. IOP measured at 14 mmHg OD and 12 mmHg OS (Table 1). The patient was pseudophakic OD and had grade 1+ nuclear sclerotic cataract OS. His topical medications included latanoprost (Falcon), timolol (Rising), and pilocarpine 2% (Sandoz). Sixty eyedrops of each medication were placed into one 10 mL bottle and the patient used the compounded solution twice a day. After 2 weeks use, the IOPs were measured at 10 mmHg OD and 10 mmHg OS. This reduction persisted at 3 months and the pressures were 10mmHG in both eyes.
|
Case |
OD |
OS |
||||||
|
Visual Acuity |
Intraocular Pressure (mmHg) |
Cup to Disc Ratio |
Central Cornea Thickness (µm) |
Visual Acuity |
Intraocular Pressure (mmHg) |
Cup to Disc Ratio |
Central Cornea Thickness (µm) |
|
|
1 |
20/30 |
14 |
0.9 |
516 |
20/20 |
12 |
0.75 |
485 |
|
2 |
CF 2 ft. |
14 |
0.9 |
493 |
CF 6 ft. |
23 |
0.9 |
509 |
|
3 |
20/20 |
14 |
0.75 |
561 |
20/20 |
14 |
0.85 |
562 |
|
4 |
CF |
36 |
0.6 |
561 |
20/20 |
15 |
0.3 |
562 |
Table 1: Baseline patient characteristics. CF = Count Fingers.
Case 2
An 82-year-old pseudophakic male with a history of glaucoma and diabetes status post cataract surgery both eyes presented for follow up visit. He had previously failed glaucoma surgery and refused any additional surgery. IOPs were 14 mmHg OD and 23 mmHg OS (Table 1). His topical medications were dorzolamide/timolol (Hi-tech), latanoprost, and netarsudil (Aerie). Sixty eyedrops of each medication were placed into one 10 mL bottle and the patient used the bottle twice a day. After 2 weeks of use, IOP was 10 mmHg OD and 11 mmHg OS. At 3 months, IOP reduction persisted at 10 mmHg OD and 10 mmHg OS.
Case 3
A 64-year-old female with a history of glaucoma and hypertension status post laser peripheral iridotomy in both eyes presented for a follow up visit. IOP was 14 mmHg OD and 14 mmHg OS (Table 1). Patient had nuclear sclerotic cataracts graded as 1+ in both eyes, and she refused cataract or glaucoma surgery to lower pressure on less medications. Her topical medications were latanoprost, dorzolamide/timolol, and pilocarpine 2% (Sandoz). Sixty eyedrops of each medication were placed into one 10 mL bottle and the patient used the solution twice a day. After 2 weeks of use, IOPs was 10 mmHg OD and 10 mmHg OS. The patient reported better compliance and reduced ocular erythema. At 3 months, IOP was 13 mmHg OD and 10 mmHg OS.
Case 4
A 45-year-old male with a history of glaucoma and central retinal vein occlusion OD presented for a follow up visit. He had a past surgical history of retinal detachment repair and retinopexy with silicone oil (and subsequent oil removal) and cataract extraction with Ahmed valve placement OD 14 months earlier. His ocular medications were latanoprost, dorzolamide-timolol OU and brimonidine OU, all twice daily. He admitted he ran out of medications and had poor compliance. IOP was 36 mmHg OD and 15 mmHg OS (Table 1).
Gonioscopy showed open angles in both eyes visible to sclera spur, and silicone droplets were seen in the superior angle OD. Sixty eyedrops of each medication were placed into one 10 ml bottle and the patient used the compounded solution twice daily (Figure 1). After 2 weeks use, IOP was 15mmHg OD and 13mm Hg OS. There was minimal conjunctival erythema and the patient reported better compliance with the medications. After 3 months of compounded therapy, the IOPs was 10 mmHg OD and 10 mmHg OS.
 Figure 1: Picture of 10ml plastic squeezable dropper bottle from Bleiou.
Figure 1: Picture of 10ml plastic squeezable dropper bottle from Bleiou.
Discussion
After the initial 2 weeks of using the compounded solution of prescription topical glaucoma treatments, all patients exhibited a trend toward decreased IOP, and this trend persisted for the majority patients at 3-month follow-up (Figure 2). Mean IOP reduction in the left eye was significant (p=0.031) after 3 months of compounded solution use when compared with baseline (Figure 2B). The utilization of the combination therapy was more effective at lowering IOP than administration of each of the medications separately. Further, this method allows for the use of personalized medications that are accessible by patients and covered by insurance. No adverse events were noted. Patients reported better compliance and reduced hyperemia. Given that none of the doses were changed, it is reasonable to assume that the compounded therapy was more effective because patients demonstrated increased persistency of use. Adherence rates in glaucoma are low compared to the treatment of other chronic illnesses [5]. Use of one medication twice daily reduces user error, saves time, and is easier to adhere to than several individual drugs. Compounding is also cost-effective, allowing patients more time between refilling medications. Patients are also exposed to fewer preservatives, which can result in eyelid erythema, follicular hyperplasia, conjunctival hyperemia, and dry-eye like symptoms [4,6].
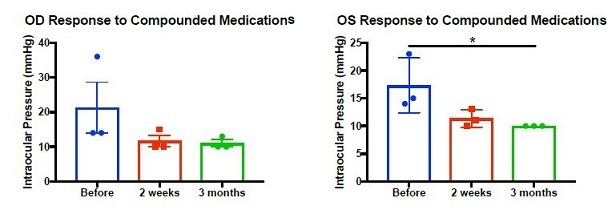
Figure 2: IOP was measured at baseline (blue) and after 2 weeks (red) and 3 months (green) of compounded medication use in (A) right eye and (B) left eye. (A) Kruskal-Wallis: p=0.074, Before vs 2 weeks: p=0.14, Before vs 3 months: p=0.094. (B) ANOVA: p=0.036, Before vs 2 weeks: p=0.67, Before vs 3 months: p=0.031. * p < 0.05.
Peer-reviewed literature has demonstrated that a clinically relevant therapeutic effect can be achieved by using fractional doses of topical medications. Pharmacodynamic effect similar to conventional eyedrop quantities has been achieved using microvolumes of 2 to 5 microliters [7]. Our report is in line with these findings, and we observed reduced hyperemia and improved therapeutic effect with smaller doses of each individual solution. Moreover, most ophthalmic solutions require the administration of one drop followed by a 5-minute period before use of the next agent. Compounding therapy eliminates this need. Compounded medications are commonly used globally, and they provide personalized medicine in which patients are treated with microvolume amounts of the specific medications that provide disease management [8].
The medications combined in this study were already being used by each patient concurrently in separate bottles. Moreover, several medications of the compounded solution are already being compounded commercially by pharmacies such as IprimisRx. The benefit of increased ease of use and ensuring that all medications are being utilized outweighs the fact that twice-daily dosing deviates from ideal dosing schedule of each individual component. For example, though latanoprost once daily has been demonstrated to be more effective than twice-daily administration, it has been shown that more frequent administration has a similar effect on IOP reduction [9]. Given the IOP reduction observed in this study, future studies that examine the galenic formulations in the specific combinations tested here are warranted.
Despite the presence of exciting therapeutic advancements in the field, the majority of patients who suffer from glaucoma worldwide will not be able to afford them. Medication compounding has the potential to significantly reduce cost to patients, increase medication access, and improve compliance, especially in resource-poor areas. When glaucoma patients were surveyed about their attitudes about novel glaucoma delivery approaches, they rated triple combination eyedrops and micro-dosing eye sprays as the two they would be most likely to accept [10]. This suggests that microvolume dosing of individual medications via a compounded eyedrop solution would be viewed favourably by the target population.
The reported compounding therapy was limited to topical solutions, and compounding of topical suspensions such as brinzolamide was not attempted and may not be as efficacious. Here, we demonstrate that personalized compounding of topical ophthalmic medications can be safe when performed in the office setting under sterile conditions. However, it remains to be determined if there is an increased risk of infection long-term compared to compounding performed in a sterile manner at a compounding pharmacy. None of the patients developed infection or required discontinued use of their compounded medication. Despite the small sample, this report provides evidence that compounding ophthalmic glaucoma therapy with all glaucoma agents with preservatives in one bottle can be more effective than individual administration at reducing IOP and is better tolerated by patients. This method should be considered and further investigated in all patients that require multiple topical agents to manage their illness, reduce ocular side effects, and reduce costs. Further studies are needed to assess long term efficacy, risks, benefits, and regulations.
Source Of Support
None.
Presentation at a Meeting
None.
Conflict of Interest
None.
References
- Pascolini D, Mariotti SP (2010) Global estimates of visual impairment: 2010. Br J Ophthalmol 96: 614-618.
- Gordon MO, Kass MA (1999) The Ocular Hypertension Treatment Study: design and baseline description of the participants. Arch Ophthalmol 117: 573-583.
- Mantravadi AV, Vadhar N (2015) Glaucoma. Prim Care 42: 437-449.
- Iester M, Telani S, Frezzotti P, Motolese I, Figus M, et al. (2014) Ocular surface changes in glaucomatous patients treated with and without preservatives beta-blockers. J Ocul Pharmacol Ther 30: 476-481.
- Meier-Gibbons F, Berlin MS, Toteberg-Harms M (2019) Influence of new treatment modalities on adherence in glaucoma. Curr Opin Ophthalmol 30: 104-109.
- Lopes NLV, Gracitelli CPB, Chalita MR, de Faria NVL (2019) Ocular Surface Evaluation After the Substitution of Benzalkonium Chloride Preserved Prostaglandin Eye Drops by a Preservative-free Prostaglandin Analogue. Med Hypothesis Discov Innov Ophthalmol 8: 52-56.
- Pasquale LR, Lin S, Weinreb RN, Tsai JC, Kramm RL, et al. (2018) Latanoprost with high precision, piezo-print microdose delivery for IOP lowering: clinical results of the PG21 study of 0.4 microg daily microdose. Clin Ophthalmol 12: 2451-2457.
- AlKhatib HS, Jalouqa S, Maraqa N, Ratka A, Elayeh E, et al. (2019) Prevalence, determinants, and characteristics of extemporaneous compounding in Jordanian pharmacies. BMC Health Serv Res 19: 816.
- Linden C, Alm A (2001) The effect on intraocular pressure of latanoprost once or four times daily. Br J Ophthalmol 85:1163-1166.
- Wang BB, Lin MM, Nguyen T, Turalba AV (2018) Patient attitudes toward novel glaucoma drug delivery approaches. Digit J Ophthalmol 24: 16-23.
Citation: Laroche D, Mike E, Ng C (2021) Personalized Glaucoma Medication Compounding Reduces Intraocular Pressure and May Increase Adherence and Affordability. J Ophthalmic Clin Res 8: 085.
Copyright: © 2021 Daniel Laroche, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

