
RE-PERG as a Diagnostic Tool for Neuroenhancement Studies in Primary Open Angle Glaucoma: A Pilot Study
*Corresponding Author(s):
Alberto MavilioASL [Local Health Authority] Of Brindisi, Italy
Email:a.mavilio@gmail.com
Abstract
Purpose: To evaluate the ability of RE-PERG to detect metabolic variations of the retinal ganglion cells (RGC) after administration of a commercially available fixed oral combination of Citicoline and Homotaurine (C+H, Neuprozin®) in primary open-angle glaucoma (POAG).
Methods: Longitudinal, interventional case-control study on 36 eyes of 36 POAG patients controlled with topical treatment and stable visual field (deteriorating by <1 dB/year). Patients were randomly divided into two groups after the first ophthalmic evaluation (T0): 18 NP (patients continued topical therapy and took the fixed combination C+H for 4 months) and 18 age and gender-matched GLA (patients continued only the current topical treatment). Four months later, a second follow-up examination (T1) was scheduled for the two groups. The effects of taking C+H were assessed through the RE-PERG, of which the amplitude, the phase standard deviation (SD Ph) and circular phase standard deviation (cSD°) of the second harmonic were calculated, along with PMI (Phase Magnitude Index). As a secondary outcome, three visual field parameters, MD, pattern standard deviation (PSD) and Visual Field Index (VFI), were also evaluated.
Results: As for RE-PERG, no variations were found in the GLA group. In the NP group, no significant amplitude variations were observed; at T1, the SDPh, and the cSD° were significantly reduced compared to T0 (0.22±0.05 vs 0.32±0.07, p=0.0002; 12.61±2.69 vs 18.30±4.36, p=0.0002, respectively), and PMI was significantly increased (0.81±0.37 vs 0.31±0.31, p=0.0003). No significant variation in the visual field parameters was observed.
Conclusion: RE-PERG can detect variations in the metabolic status of the RGCs in glaucomatous patients after oral intake of a fixed combination of C+H in addition to topical treatment. RE-PERG can be a valid tool in neuroenhancement studies, representing a biological marker of neuronal performance.
Keywords
Neuroenhancement; Citicoline; Homotaurine; Glaucoma; Pattern electroretinogram; Re-perg
Introduction
Glaucoma is a progressive optic neuropathy induced by the loss of retinal ganglion cells, and in particular, their axons. The biggest known risk factor for glaucoma is intraocular pressure (IOP) [1].
However, it has been observed that the condition also progresses in the presence of good IOP control, suggesting that the condition is caused by multiple factors. Furthermore, there is evidence that the neurodegeneration spreads from the Retinal Ganglion Cells (RGCs) to central neuron stations, such as lateral geniculate bodies, via trans-synaptic degeneration [2-5].
For these reasons, the approach to the condition has become similar to that reserved by neurologists for neurodegenerative conditions, such as Parkinson’s or Alzheimer’s diseases. There are growing indications for using substances with presumed neuroactive or neuroprotective action in glaucoma [6-11]. However, it is necessary to objectively verify the effectiveness of such substances, which are supposed to act on the function of surviving ganglion cells that have not yet been affected by apoptosis.
Pattern electroretinogram, PERG, is an objective test of the RGCs function, useful for the early diagnosis of glaucoma and for monitoring the condition, able to prevent deterioration which is subsequently discovered by other methods [12,13]. Furthermore, it can show improvements following the reduction of IOP [14,15]. The steady-state pattern electroretinogram appears more sensitive than the transient PERG since it subjects the RGCs to more significant metabolic stress [16]. Porciatti has developed a paradigm known as PERGLA (PERG for glaucoma screening), showing high specificity and sensitivity in diagnosing glaucoma [17,18].
The usually studied parameter is the amplitude of the second harmonic. This parameter, however, may be influenced by non-specific causes, such as cataracts and myopia. It has recently developed a variant of the steady-state PERG, called RE-PERG (i.e. Repeated-PERG), which consists of intra-test repetition, without pauses, of 5 blocks of events [19]. In this way, phase variability is studied. The authors observed that the phase standard deviation is low in normal subjects but increases in glaucomatous patients. The study of this parameter has proven helpful in distinguishing early-stage glaucoma from normal subjects; also, it is not influenced by cataracts and myopia [20,21]. In addition, although with other procedures, other authors have found higher phase variability of steady-state PERG in glaucoma patients [22-24].
Citicoline has proven useful in pathological conditions of both the central nervous system and ophthalmology. In particular, it has been used in amblyopia, anterior ischemic optic neuropathy, and in glaucoma, where it has shown neuroprotective potential following both intramuscular and oral administration [25].
Homotaurine is an inhibitor of amyloid-β deposition, used in experiments on Alzheimer’s disease [26]. Since amyloid-β deposition has also been proven in the retinal ganglion cells in glaucoma; there is a rationale for assessing its potential neuroprotective action in cases of glaucoma [27].
Currently, there is a distinction between maintaining RGCs survival (neuroprotection), protecting or rebuilding RGCs connections in the retina and the brain (neuroregeneration), and enhancing RGCs function (neuroenhancement) [7,28]. Therefore, RGCs metabolic improvement in the short term is considered a neuroenhancement effect, which can be assessed by electrophysiological tests. In contrast, slowing down or preventing RGCs death in the long term is a neuroprotective effect, which can be assessed by perimetry and optic disc evaluation. It is reasonable to think that, when the effectiveness of a potential neuroprotective drug is verified, the first step should be evaluating the neuro-enhancing effect, and subsequently, its neuroprotective action.
The purpose of this pilot study was to verify the ability of RE-PERG to detect the short-term effect of a compound available on the market containing 500 mg of ultra-pure Citicoline (Cognizin®) and 50 mg of Homotaurine (C+H, Neuprozin®), on RGCs metabolic dysfunction in a population of subjects affected by early-stage glaucoma.
Materials and Methods
Trial design
The study was conducted according to recommendations of the Helsinki declaration (revision 2000, Edinburg) and the Italian Good Clinical Practice legislation (DM 15 Luglio 1997 and modifications). It is an ancillary study of a multicenter study approved by the Ethics Committee of the Fondazione IRCCS Policlinico San Matteo of Pavia (prot. 2015000565), registered on ClinicalTrials.gov (NCT04422743). The pilot study was approved by the Ethics Committee of ASL Brindisi and the University of Bari; all patients signed a written consent form.
Since 2005, we have developed, at the ASL of Brindisi and at the University of Bari, a new test, called RE-PERG, aimed to study the metabolic state of the retinal ganglion cells in glaucoma. We showed that this test is specifically altered in glaucoma and not influenced by cataracts and myopia. We supposed that it could be a valid tool to evaluate the efficacy of neuroprotective drugs. Therefore, patients enrolled at the ASL of Brindisi and the Eye Clinic of the “Aldo Moro” University of Bari (Italy) for the primary multicenter study were submitted for further examination with the RE-PERG method.
The following inclusion criteria were applied: diagnosis of primary open-angle glaucoma (POAG) at least 3 years earlier, visual acuity > 0.7 (7/10) decimals, refractive errors <5 D (spherical) and <2D (toric), transparent dioptric media (cornea and lens), IOP under control (<18mmHg, morning value) with beta-blockers or prostaglandins as a single or combined treatment (fixed or unfixed), stable IOP <18mmHg in the last 2 years, stable and unvaried topical treatment in the last 6 months, early glaucoma (stage I-II following the GSS2 [27], MD ranging from -2.5 to -6), stable defect of the visual field in the last 2 years (no more than -1dB/year in MD), at least two reliable visual fields (Humphrey 24-2 SITA Standard) per year in the last 2 years, changes in the electrophysiological parameters (PERG) associated with glaucoma, written consent for participation in the study’s procedures (see Detailed study procedures) and the use of data in an anonymous form.
The following exclusion criteria were applied: ocular hypertension with normal optic nerve and visual field; closed angle glaucoma; congenital glaucoma; secondary glaucoma; normal-tension glaucoma, prior history of uveitis, scleritis and herpes infections, pregnancy and breastfeeding, contra-indications to the intake of Citicoline and/or Homotaurine, contra-indications to beta-blockers and similar prostaglandins, topical treatment with Brimonidine or fixed combination (with timolol or brinzolamide), treatment with pilocarpine and aceclidine, sole treatment or fixed combination, systemic or topical treatment with another neuroprotector in the 4 months before signing the consent form, systemic treatments that may influence patients’ performance in the visual field examination (e.g. sedatives), glaucomatous scotoma in the central 10 degrees, any condition that could limit the patient’s ability to participate in the study, other ocular causes resulting in visual field and PERG changes, such as cataracts, myopic chorioretinopathy, maculopathy, retinal vascular occlusion, diabetic retinopathy, other systemic causes that result in visual field and PERG alterations, such as neurodegenerative disorders (Alzheimer’s disease, Parkinson’s disease, ALS, MS) or pituitary disorders, antidepressant-antipsychotic medication, cerebral ischemia in the last 2 years, any change in the topical treatment in the six months before signing the consent form or during the study, loss of IOP control during the study (i.e. IOP> 21 mmHg), simultaneous participation in another clinical study, prior glaucoma surgery or vitreoretinal surgery, cataract surgery in the last 6 months, laser treatment for glaucoma in the last 5 years.
Following the recruitment examination, we enrolled 36 patients who were divided randomly into two groups of eighteen each (one eye per patient, chosen randomly) in a single-blind study:
Neuprozin® (NP) group
(T0) Recruitment with the acquisition of the baseline data; the patients continue their current topical therapy and start to take one tablet per day of the fixed supplement C+H for 4 months
(T1) follow-up examination
Non-Neuprozin® (GLA) group
(T0) Recruitment with the acquisition of the baseline data: the patients continue their current topical therapy
(T1) follow-up examination
At each examination (T0-T1) the patients underwent the following routine procedures: complete ophthalmic examination with visual acuity, intraocular pressure (IOP) measurement utilising Goldmann applanation tonometry, anterior and posterior segment evaluation, RE-PERG, visual field examination (24-2 test, SITA Standard strategy).
The methods for RE-PERG and visual field assessment are the same used in previous studies and are described as follows
RE-PERG: It was performed with an instrument available on the market (RETIMAX Advanced ver. 4.3; CSO, Florence, Italy). The stimulus, consisting of a square pattern made up of horizontal high temporal frequency bars (1.63 cycles/degree), was modulated in counter-phase at 7.5 Hz (15 inversions per second) electronically generated by a high-resolution Plasma monitor (nominal contrast: 99%; average luminance: 60 cd / m2 Subtended field: central 30°).
The subjects, seated at a distance of 57 cm with correction of the refraction error and undilated pupil, were examined with common 9mm-diameter Ag / AgCl skin electrodes placed on the lower lid. A similar electrode, placed on the lower lid of the contralateral eye, i.e. the eye that was not being stimulated, was used as a reference. The electrode impedances were below 3 kΩ. The responses were amplified (50,000), filtered with a band pass filter (1-30 Hz) and sampled at 3.84 kHz with 24bit resolution, with automatic rejection of the artefacts. The average response was analysed 5 times per session to study the intrinsic phase variability.
Once the second harmonic of the frequency spectrum had been isolated, the amplitude (in µV) and the phase (in radians) of the signal were measured with the Fourier transform.
Since the phase is an angular value, in addition to the standard deviation, we also calculated the circular standard deviation in degrees (cSD°). To verify if the phase variability depends on the signal-to-noise ratio (SNR), we measured this data. We calculated SNR as the ratio between the root mean square of magnitude and the root mean square of the noise. So the noise was on average 0.09+-0.02, and we excluded traces with SNR dB<3. Finally, we studied the correlations between all the variables.
In this study we also introduce a new cumulative RE-PERG index, PMI (Phase Magnitude Index) which resumes all the data relative to amplitude and phase, according to the following formula:
((LN(Amp)-0,8)+2,6)^0,2)*(LN(rad)+0,7)^2)
Where Amp is the mean amplitude and rad is the mean phase rad value. Thus, amplitudes and phase variability values assume logarithmic progression, and therefore the reduced amplitudes produce lesser effects on the PMI the more minor the phase variability. According to our normative values, the cut-off is equal to 1.35. Lower values indicate dysfunction even with apparently normal amplitudes, and higher values indicate normality even with reduced amplitudes because they are accompanied by physiological signal variability. Consequently, PMI is a single value able to express retinal ganglion cell dysfunction and is expected to increase after effective neuroprotective therapy.
Visual Field: this was performed with an instrument available on the market (Humphrey Field Analyzer, model 745i II, Carl Zeiss Meditec AG, Jena, Germany), using the 24-2 test with the SITA Standard strategy, with adequate optical correction of presbyopia. Tests with fixation losses >20% and false positive and false negative errors >15% were eliminated [29]. The three global parameters of the visual field (MD, mean defect, PSD, pattern standard deviation and VFI, visual field index) were evaluated.
Statistic Analysis
Thirty-six subjects guaranteed a level of significance alpha = 0.05 and p <0.05 to have 90% power to detect a variation of 25% of the parameters considered, taking into account that the test-retest variability does not exceed 20% (Coefficient of variation). The statistical analysis was performed with a paired Student’s T-test for single variables, and a chi-square test for discrete variables; the differences were considered significant for values of p<0.05; (Medcalc® 20.111). Circular statistics and circular distribution of the phase were performed employing Oriana® Ver. 4.02
Results
As for general demographics, there was no statistically significant difference between GLA and NP groups for age and male percentage (64.5±8.36 vs 60.4±7.6 years, p=0.48 Student’s T-test, and 55.5% vs 61.1%, p= 0.73, chi-square test, respectively). Specific population data and study results are summarised in table 1. There was, at baseline, no statistical difference for any of the parameters considered in the two groups.
|
|
T0 |
T1 |
|
||||||
|
|
GLA (n.18) |
NP (n.18) |
GLA (n.18) |
NP (n.18) |
TO vs T1 (NP group) |
||||
|
|
Mean |
SD |
Mean |
SD |
Mean |
SD |
Mean |
SD |
p value (paired Student’s T-test) |
|
IOP |
16 |
1.53 |
15.78 |
1.52 |
15.72 |
1.13 |
16.28 |
1.32 |
0.33 |
|
MD |
-4.39 |
2.52 |
-4.56 |
1.89 |
-4.69 |
2.82 |
-4.21 |
2.44 |
0.57 |
|
VFI |
90.44 |
7.01 |
89.28 |
5.46 |
90.06 |
7.01 |
89.5 |
8.54 |
0.14 |
|
PSD |
4.23 |
0.97 |
4.60 |
1.25 |
4.12 |
1.89 |
4.64 |
2.24 |
0.96 |
|
AMP |
1.33 |
0.15 |
1.35 |
0.17 |
1.34 |
0.17 |
1.35 |
0.22 |
0.96 |
|
SD_Ph |
0.29 |
0.09 |
0.32 |
0.08 |
0.31 |
0.12 |
0.22 |
0.05 |
0.0002 |
|
cSD° |
16.74 |
5.52 |
18.3 |
4.37 |
17.7 |
6.49 |
12.61 |
2.69 |
0.0002 |
|
PMI |
0.49 |
0.46 |
0.31 |
0,3052 |
0.42 |
0.33 |
0.81 |
0.37 |
0.0003 |
|
SNR |
15.67 |
4.26 |
17,.8 |
5.45 |
16.48 |
3.36 |
17.16 |
4.47 |
0.58 |
Table 1: Clinical data of glaucoma patients (GLA) and patients with NP (NP) patients at T0-T1. Abbreviations: mean Amplitude (Amp), the circular standard deviation in degree (cSD°), Phase-Magnitude Index (PMI), intrinsic variability of the phase (SD Phase), signal-to-noise ratio (SNR), Intraocular Pressure (IOP), visual field index (VFI), mean deviation (MD), pattern standard deviation (PSD) e of Visual Field. Units of measurement are in parentheses. Significant p values are in bold characters.
As for RE-PERG, no variations were found in the GLA group. In the NP group, no significant amplitude variations were observed; at T1, the SDPh and the cSD° were significantly reduced compared to T0 (0.22±0.05 vs 0.32±0.07, p=0.0002; 12.61±2.69 vs 18.30±4.36, p=0.0002, respectively; an example in figure 1) and PMI was significantly increased (0.81±0.37 vs 0.31±0.31, p=0.0003). The difference in PMI between GLA and NP groups is also shown in figure 2 as cumulative frequency distribution. No significant variation in the visual field parameters was observed.
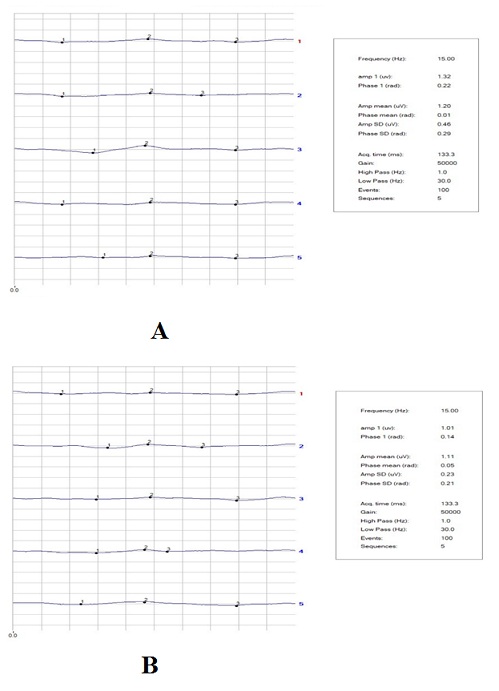 Figure 1: A-B. Intersession RE-PERG of an NP patient at T0 (A) and T1 (B). Intrinsic variability of the phase shows in T1 a reduced value concerning baseline (T0). Magnitude changes are not significant between sessions.
Figure 1: A-B. Intersession RE-PERG of an NP patient at T0 (A) and T1 (B). Intrinsic variability of the phase shows in T1 a reduced value concerning baseline (T0). Magnitude changes are not significant between sessions.
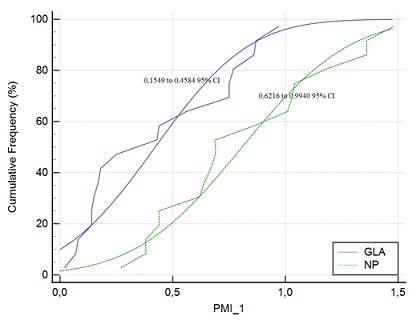 Figure 2: The cumulative frequency distribution plot shows differences in Phase-Magnitude Index (PMI) values between groups at 4 months from the treatment, showing an improvement of PMI in the NP group with 95% confidence intervals with no overlap between groups.
Figure 2: The cumulative frequency distribution plot shows differences in Phase-Magnitude Index (PMI) values between groups at 4 months from the treatment, showing an improvement of PMI in the NP group with 95% confidence intervals with no overlap between groups.
Discussion
This trial showed improvement in the neurological performance of the inner retina, as shown by the RE-PERG examination, after the oral intake of C+H in the NP group, as a reduction of SD_Ph and cSD°; such improvement is also confirmed by the increase of the cumulative index PMI.
Although IOP is the main risk factor for the development and progression of glaucoma, it is also known that other, not IOP-related, factors, can contribute to RGCs degeneration, such as oxidative stress, glutamate excitotoxicity, ischemia or impaired microcirculation, mitochondrial dysfunction, autoimmune dysregulation, activation of neurotrophic growth factor, activation of microglia and astrocytes, protein misfolding [30,31].
Therefore, there is a growing demand for not only clinical evidence about the neuroprotective effect of various drugs in glaucoma but also reliable diagnostic tools able to measure the effect.
Citicoline is an organic compound indispensable for the synthesis of the phospholipids of the cell membrane. When taken orally, citicoline is quickly absorbed and converted into cytidine and choline by the action of the phosphodiesterases present in the cells of the intestinal epithelium. Its metabolites reach the brain approximately 30 minutes after administration, while their level in the blood increases slowly, peaking 6 hours after oral intake. The conversion in cytidine and choline also happen following intravenous and intramuscular administration [32].
In the central nervous system, Citicoline encourages an increase in the biosynthesis of phosphatidylcholine (PC); choline is used for the synthesis of the neurotransmitter acetylcholine. The choline that is released by citicoline can, in addition, be metabolised in glutathione, a powerful antioxidant that is endogenous in the brain. Glutathione plays a neuroprotective role, reducing lipid peroxidation [33].
In amblyopia, citicoline has been proven to improve visual acuity, contrast sensitivity, visually evoked responses and the effects of part-time occlusion, at least temporarily, in patients with amblyopia during the plasticity of the visual system [34,35]. In non-arteritic ischaemic optic neuropathy, patients treated with citicoline showed an improvement in the PERG, PEV and visual acuity with the administration of oral Citicoline [36]. In glaucoma, citicoline improved the visual field and electro-functional parameters [11,37]. It has been proven to be effective also by oral and topic administration [38,39].
Homotaurine (3-aminopropanesulfonic acid) is a small natural compound subsequently synthesised chemically and introduced to the pharmaceutical market with the name tramiprosate [40]. Homotaurine has proven to be able to interfere with numerous biological pathways, in experimental models both in vitro and in vivo, and to possess neuroprotective properties via more than one mechanism [41]. Its neuroprotective effects in glaucoma could be derived from its action as an agonist of the GABA-A receptor and as an inhibitor of the NMDA selector when abnormally active [42].
Homotaurine, together with other compounds, has been administered in POAG, showing an improvement in the visual field, light sensitivity, contrast sensitivity and a better quality of life in a study and an increase in PERG amplitude and foveal sensitivity in another study [43,44].
The same formulation administered in the present work has been shown to increase the total score of the contrast sensitivity test and the quality of life in patients with POAG [45].
It has been shown that following a reduction in IOP, the perimetry test shows an improvement in retinal sensitivity, and the PERG shows a significant increase in amplitudes [14,15]. After IOP reduction, the RGCs dysfunction is likely to be reversed; this reversal, within certain limits, causes a decrease in the MD of the visual field and the PERG amplitude [46]. But, unlike PERG, an objective test, the visual field is a psycho-physical test influenced by the patient’s variable cooperation. This is the reason why PERG is a more reliable tool for the evaluation of potential neuroprotective drugs.
PERG has proven to be an effective, non-invasive, objective test for measuring the function of the RGC [47,48]. This testing method has high specificity and sensitivity values, with both transient and steady-state stimuli. It has been shown that, with the progress of the condition, a linear correlation between the reduction of the amplitude/latency delay of the PERG and the reduction of RNFL thickness of the OCT and the MD of the perimetry test is present [49-52].
The PERG explores the central 24°-30° of the retina and is, therefore, affected by the presence of a macular dysfunction or by the opacity of the media. The steady-state method appears to detect the dysfunction of the ganglion cells with higher specificity, probably because a high temporal frequency stimulus subjects these cells to stress to the extent that shows a greater susceptibility compared to healthy cells [16].
Fourier’s analysis of the signal obtained with a steady-state stimulus, however, entails the risk of numerous artefacts if rigid testing settings are not complied with [53]; but, at the same time, it is predictive, compared to other common investigations for the early diagnosis of glaucoma [50].
Porciatti has suggested an optimised paradigm for glaucoma screening (PERGLA), showing high test-retest repeatability, sensitivity and specificity.17 The diagnostic accuracy of the protocol has been confirmed by various authors [54,55]. One advantage lies in the possibility of using skin electrodes, which minimise the test’s invasive nature and make it user-friendly, and ideal for screenings. Furthermore, it shows alterations in the RGC before they are detected by the perimetry test and OCT [56,57]. Even this paradigm is not completely free of possible false positives, especially in the presence of deterioration of the lens [58].
Recently, our study group highlighted a new parameter of the steady-state PERG, which until now had been underestimated, represented by the intrinsic phase variability of the bio-electrical signal [19,20].
The PERG is a particularly sensitive test, which is why it is susceptible to numerous variables that can compromise the test result, reducing the amplitude of the bio-electric response to the stimulus. For example, a deterioration of the lens, such as an early cataract, or even refractive correction that is inadequate for the working distance, suffices to obtain low-amplitude responses that can be erroneously interpreted as pathological.
To obviate these limitations, Bach suggests a solution by evaluating the amplitude of the intra-individual and intra-session signal through a PERG ratio we found out that the intrinsic phase variability is very low in all normal subjects [19,59-61].
In brief, we observed that, by repeating 5 steady-state PERG sessions in quick succession, the value of the phase delay of each block is almost identical, or in any case with very low variability in all normal subjects. This intrinsic variability is, instead, significantly increased in the presence of internal retinal dysfunction [19].
To demonstrate that intrinsic phase variability is an independent variable concerning lower SNR, at the list for SNR >3, we have proven that early cataracts do not alter the intrinsic phase variability, significantly improving PERG specificity in the diagnosis of glaucoma [20].
Also, in the present research, we found that SD_Ph and circular variable are not correlated with the noise level, at the list for SNR>3.
We recently proved that in subjects with high myopia, in whom the diagnosis of glaucoma is complicated by the presence of morphological and functional alterations, our diagnostic procedure, called RE-PERG, makes it possible to identify the pathology by assessing the intrinsic phase variability, which is significantly higher only in the glaucoma group [21].
To date, the objective assessment of the bioelectric activity of the inner retina, through the study of the PERG, has been exclusively entrusted to the amplitude of the signal; this datum, however, is susceptible to numerous variables such as the opacity of the media.
The morphology of the wave obtained with the PERG is an aspect - to date overlooked - of the quality of the bioelectric potential due to the signal-to-noise ratio (type or position of the electrodes, gain, etc.) and directly to the quality of the signal. As it results from the average of the acquired events, the morphology depends strictly on the perfect repeatability of the events.
By increasing the temporal frequency of the stimulus, as happens with the steady-state technique, the obtained peaks gradually come closer until they form, under normal conditions, a sinusoidal wave. Therefore, if the events are not perfectly repeatable, the morphology of this wave will be modified. The intrinsic phase variability of the RE-PERG is a direct expression of the morphology of the waves; this is why it will be much lower under normal conditions and high when the variability of the electrophysiological events is pathologically high.
In other words, in the presence of dysfunction, the RGCs response is not perfectly synchronous with the stimulus, which explains the high intrinsic phase variability that is never seen in healthy subjects. This parameter, detected by the RE-PERG, could, therefore, become a biological marker of neuronal performance [20].
The present study was not aimed to demonstrate a neuroprotective effect of the compound Neuprozin®; such a study requires longer follow-up, using as reference parameters perimetry and the Optical Coherence Tomography of the optical nerve and the RGCs. In the short term, as stated above, it is only possible to determine a metabolic improvement of the RGCs, which is a neuroenhancement effect. This is the object of the multicenter study of which this is an ancillary work. Our outcome suggests that RE-PERG can be used as a diagnostic tool for monitoring the variation of the metabolic state of RGCs after the administration of potentially neuroprotective drugs.
The limit of the present study is undoubtedly the reduced number of patients being examined; however, it retains its validity as a pilot study which needs to be confirmed using a larger sample of patients.
In addition, since we administered a fixed combination of citicoline and homotaurine, we cannot understand if homotaurine provides an additive effect to citicoline or if our results are due only to citicoline.
Conclusion
Our data found a positive effect of Neuprozin® on inner retina function examined by electroretinogram. The interesting thing about the study is the use of a new and promising technique to evaluate PERG, the RE-PERG: this technique has shown sensitivity to change, which makes the method a precious objective assessment tool in this field. In particular, the intrinsic phase variability of the RE-PERG could become a biological marker of neurological performance and, therefore, a new parameter to use in glaucoma studies to detect and follow the efficacy of new therapeutic approaches.
Conflict of Interest Statement
This study received funding from FB Vision S.p.A. (San Benedetto del Tronto, Italy). The funder was not involved in the study design, collection, analysis, and interpretation of data, the writing of this article or the decision to submit it for publication. All authors declare no other competing interests.
References
- Weinreb RN, Khaw PT (2014) Primary open-angle glaucoma. Lancet 363: 1711-1720.
- Heijl A, Bengtsson B, Hyman L, Leske MC (2009) Natural history of open-angle glaucoma. Ophthalmology 116: 2271-2276.
- Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol 130: 429-440.
- Ederer F, Gaasterland DA, Dally LG, Kim J, VanVeldhuisen PC, et al. (2014) The Advanced Glaucoma Intervention Study (AGIS): 13. Comparison of treatment outcomes within race: 10-year results. Ophthalmology 111: 651-664.
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, et al. (2003) Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol 121: 48-56.
- Parisi V, Miglior S, Manni G, Centofanti M, Bucci MG (2006) Clinical ability of pattern electroretinograms and visual evoked potentials in detecting visual dysfunction in ocular hypertension and glaucoma. Ophthalmology 113: 216-228.
- Weinreb RN (2007) Glaucoma neuroprotection: What is it? Why is it needed? Can J Ophthalmol 42: 396-398.
- Quaranta L, Bettelli S, Uva MG, Semeraro F, Turano R, et al. (2003) Effect of Ginkgo biloba extract on preexisting visual field damage in normal tension glaucoma. Ophthalmology 110: 359-362.
- Falsini B, Porciatti V, Bolzani R, Marchionni A (1991) Spatial-frequency-dependent changes in the human pattern electroretinogram after acute acetyl-L-carnitine administration. Graefes Arch Clini Exp Ophthalmol 229: 262-266.
- Falsini B, Marangoni D, Salgarello T, Stifano G, Montrone L (2009) Effect of epigallocatechin-gallate on inner retinal function in ocular hypertension and glaucoma: a short-term study by pattern electroretinogram. Graefes Arch Clin Exp Ophthalmol 247: 1223-1233.
- Parisi V (2005) Electrophysiological assessment of glaucomatous visual dysfunction during treatment with cytidine-5′-diphosphocholine (citicoline): a study of 8 years of follow-up. Doc Ophthalmol 110: 91-102.
- Bode SFN, Jehle T, Bach M (2011) Pattern electroretinogram in glaucoma suspects: new findings from a longitudinal study. Invest Ophthalmol Vis Sci 52: 4300-4306.
- Bach M, Poloschek CM (2013) Electrophysiology and glaucoma: current status and future challenges. Cell Tissue Res 353: 287-296.
- Sehi M, Grewal DS, Goodkin ML, Greenfield DS (2010) Reversal of retinal ganglion cell dysfunction after surgical reduction of intraocular pressure. Ophthalmology 117: 2329-2336.
- Sehi M, Grewal DS, Feuer WJ, Greenfield DS (2011) The impact of intraocular pressure reduction on retinal ganglion cell function measured using pattern electroretinogram in eyes receiving latanoprost 0.005% versus placebo. Vision Res 51: 235-242.
- Porciatti V, Sorokac N, Buchser W (2005) Habituation of retinal ganglion cell activity in response to steady state pattern visual stimuli in normal subjects. Investigative ophthalmology & visual science. 46: 1296-1302.
- Porciatti V, Ventura LM (2004) Normative data for a user-friendly paradigm for pattern electroretinogram recording. Ophthalmology 111: 161-168.
- Porciatti V, Ventura LM (2009) Adaptive changes of inner retina function in response to sustained pattern stimulation. Vision Res 49: 505-513.
- Mavilio A, Scrimieri F, Errico D (2015) Can variability of pattern ERG signal help to detect retinal ganglion cells dysfunction in glaucomatous eyes? Biomed Res Int 2015: 571314.
- Mavilio A, Sisto D, Ferreri P, Cardascia N, Alessio G (2017) RE-PERG, a new procedure for electrophysiologic diagnosis of glaucoma that may improve PERG specificity. Clin Ophtalmol 11: 209-218.
- Mavilio A, Sisto D, Ferreri P, Dammacco R, Alessio G (2019) RE-PERG, a new paradigm for glaucoma diagnosis, in myopic eyes. Clin Ophtalmol 13: 1315-1322.
- Lim XH, Nongpiur ME, Najjar RP, Hoang QV, Milea D, et al. (2021) Steady-State Pattern Electroretinography in Eyes with Glaucoma and High Myopia. Clin Ophthalmol 15: 4455-4465.
- Tirsi A, Gliagias V, Moehringer J, Orshan D, Tello S, et al. (2021) Pattern electroretinogram parameters are associated with optic nerve morphology in preperimetric glaucoma after adjusting for disc area. J Ophthalmol 2021: 8025337.
- Tirsi A, Orshan D, Wong B, Gliagias V, Tsai J, et al. (2022) Associations between steady-state pattern electroretinography and estimated retinal ganglion cell count in glaucoma suspects. Documenta Ophthalmologica 145: 11-25.
- Parisi V, Manni G, Colacino G, Bucci MG (1999) Cytidine-5′-diphosphocholine (citicoline) improves retinal and cortical responses in patients with glaucoma. Ophthalmology 106: 1126-1134.
- Caltagirone C, Ferrannini L, Marchionni N, Nappi G, Scapagnini G, et al. (2012) The potential protective effect of tramiprosate (homotaurine) against Alzheimer’s disease: a review. Aging Clin Exp Res 24: 580-587.
- Yin H, Chen L, Chen X, Liu X (2008) Soluble amyloid β oligomers may contribute to apoptosis of retinal ganglion cells in glaucoma. Med Hypotheses 71: 77-80.
- Chang EE, Goldberg JL (2012) Glaucoma 2.0: Neuroprotection, Neuroregeneration, Neuroenhancement. Ophthalmology 119: 979-986.
- Heijl A, Lindgren G, Olsson J (1989) The effect of perimetric experience in normal subjects. Arch Ophthalmol 107: 81-86.
- Faiq MA, Wollstein G, Schuman JS, Chan KC (2019) Cholinergic nervous system and glaucoma: from basic science to clinical applications. Prog Retin Eye Res 72: 100767.
- Gandolfi S, Marchini G, Caporossi A, Scuderi G, Tomasso L, et al. (2020) Cytidine 5-diphosphocholine (citicoline): evidence for a neuroprotective role in glaucoma. Nutrients 12: 793.
- Grieb P, Rejdak R (2006) Pharmacodynamics of citicoline relevant to the treatment of glaucoma. J Neurosci Res 67: 143-148.
- Barrachina M, Secades J, Lozano R, Gómez-Santos C, Ambrosio S, et al. (2002) Citicoline increases glutathione redox ratio and reduces caspase-3 activation and cell death in staurosporine-treated SH-SY5Y human neuroblastoma cells. Brain Res 957: 84-90.
- Pawar PV, Mumbare SS, Patil MS, Ramakrishnan S (2014) Effectiveness of the addition of citicoline to patching in the treatment of amblyopia around visual maturity: a randomized controlled trial. Indian J Ophthalmol 62: 124-129.
- Porciatti V, Schiavi C, Benedetti P, Baldi A, Campos EC (1998) Cytidine-5'-diphosphocholine improves visual acuity, contrast sensitivity and visually-evoked potentials of amblyopic subjects. Curr Eye Res 17: 141-148.
- Parisi V, Barbano L, Di Renzo A, Coppola G, Ziccardi L Neuroenhancement and neuroprotection by oral solution citicoline in non-arteritic ischemic optic neuropathy as a model of neurodegeneration: A randomized pilot study. PloS One 14: 0221313.
- Pecori Giraldi J, Virno M, Covelli G, Grechi G, De Gregorio F (1989) Therapeutic value of citicoline in the treatment of glaucoma (computerized and automated perimetric investigation). Int ophthalmol 13: 109-112.
- Ottobelli L, Manni GL, Centofanti M, Iester M, Allevena F, et al. (2013) Citicoline oral solution in glaucoma: is there a role in slowing disease progression? Ophthalmologica 229: 219-226.
- Rossetti L, Iester M, Tranchina L, Ottobelli L, Coco G, et al. (2020) Can Treatment With Citicoline Eyedrops Reduce Progression in Glaucoma? The Results of a Randomized Placebo-controlled Clinical Trial. J Glaucoma 29: 513-520.
- Messina SA, Dawson R Jr (2000) Attenuation of oxidative damage to DNA by taurine and taurine analogs. Adv Exp Med Biol 483: 355-367.
- Biasetti M, Dawson R Jr (2002) Effects of sulfur containing amino acids on iron and nitric oxide stimulated catecholamine oxidation. Amino Acids 22: 351-368.
- Tian J, Dang H, Wallner M, Olsen R, Kaufman DL (2018) Homotaurine, a safe blood-brain barrier permeable GABA A-R-specific agonist, ameliorates disease in mouse models of multiple sclerosis. Scientific reports 8: 1-8.
- Rolle T, Dallorto L, Rossatto S, Curto D, Nuzzi R (2020) Assessing the Performance of Daily Intake of a Homotaurine, Carnosine, Forskolin, Vitamin B2, Vitamin B6, and Magnesium Based Food Supplement for the Maintenance of Visual Function in Patients with Primary Open Angle Glaucoma. J Ophthalmol 2020: 7879436.
- Mutolo MG, Albanese G, Rusciano D, Pescosolido N (2016) Oral Administration of Forskolin, Homotaurine, Carnosine, and Folic Acid in Patients with Primary Open Angle Glaucoma: Changes in Intraocular Pressure, Pattern Electroretinogram Amplitude, and Foveal Sensitivity. J Ocul Pharmacol Ther 32: 178-183.
- Marino PF, Rossi GCM, Campagna G, Capobianco D, Costagliola C, et al (2020) Effects of Citicoline, Homotaurine and Vitamin E on Contrast Sensitivity and Visual-Related Quality of Life in Patients with Primary Open-Angle Glaucoma: A Preliminary Study. Group. Molecules 25: 5614.
- Ventura LM, Porciatti V (2005) Restoration of retinal ganglion cell function in early glaucoma after intraocular pressure reduction: a pilot study. Ophthalmology 112: 20-27.
- Holder GE (2001) Pattern electroretinography (PERG) and an integrated approach to visual pathway diagnosis. Prog Retin Eye Res 20: 531-561.
- Bach M, Hoffmann M (2006) The origin of the pattern electroretinogram. Principles and practice of clinical electrophysiology of vision 2006: 185-96.
- Ventura LM, Sorokac N, De Los Santos R, Feuer WJ, Porciatti V (2006) The Relationship between Retinal Ganglion Cell Function and Retinal Nerve Fiber Thickness in Early Glaucoma. Invest Ophthalmol Vis Sci 47: 3904-3911.
- Banitt MR, Ventura LM, Feuer WJ, Savatovsky E, Luna G, et al. (2013) Progressive loss of retinal ganglion cell function precedes structural loss by several years in glaucoma suspects. Invest Ophthalmol Vis Sci 54: 2346-2352.
- Hood D, Xu L, Thienprasiddi P (2005) The Pattern Electroretinogram in Glaucoma Patients with Conñrmed Visual Field Deficits. Invest. Ophtalmol Vis Sci 46: 2411-2418.
- Porciatti V, Ventura LM (2009) Physiological significance of steady-state PERG losses in glaucoma: clues from simulation of abnormalities in normal subjects. J Glaucoma 18: 535-542.
- Bach M, Meigen T (1999) Do’s and don’ts in Fourier analysis of steady-state potentials. Doc Ophthalmol 99: 69-82.
- Bowd C, Vizzeri G, Tafreshi A, Zangwill LM, Sample PA, et al. (2009) Diagnostic Accuracy of Pattern Electroretinogram Optimized for Glaucoma Detection. Ophthalmology 116: 437-443.
- Fredette MJ, Anderson DR, Porciatti V, Feuer W (2008) Reproducibility of Pattern Electroretinogram in Glaucoma Patients with a Range of Severity of Diseases with the New Glaucoma Paradigm. Ophthalmology 115: 957-963.
- Forte R, Ambrosio L, Bonavolontà P, Ambrosio G (2010) Pattern electroretinogram optimized for glaucoma screening (PERGLA) and retinal nerve fiber thickness in suspected glaucoma and ocular hypertension. Doc Ophthalmol 120: 187-192.
- Yang A, Swanson WH (2007) New Pattern Electroretinogram Paradigm Evaluated in Terms of User Friendliness and Agreement with Perimetry. Ophthalmology 114: 671-679.
- Vizzeri G, Tafreshi A, Weinreb RN, Bowd C (2010) Effect of Operator and Optica Defocus on the Variability of Pattern Electroretinogram Optimized for Glaucoma Detection (PERGLA); J Glaucoma 19: 77-82.
- Bach M, Hiss P, Rover J (1988) Check-size specific changes of pattern electroretinogram in patients with early open-angle glaucoma. Doc Ophthalmol 69: 315-322.
- Bach M (1988) Electrophysiological approaches for early detection of glaucoma. Eur J Ophthalmol 11 (suppl2): S41-S49.
- Bach M, Ramharter-Sereinig A (2013) Pattern electroretinogram to detect glaucoma: comparing the PERGLA and the PERG Ratio protocols. Doc Ophthalmol 127: 227-238.
Citation: Sisto D, Albano V, Mavilio A, Ferreri P, Rossi GCM, et al. (2022) RE-PERG as a Diagnostic Tool for Neuroenhancement Studies in Primary Open Angle Glaucoma: A Pilot Study. J Ophthalmic Clin Res 9: 106.
Copyright: © 2022 Dario Sisto, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

