
The Effect of Chromium in the Management of Diabetic Macular Edema: An Interventional Comparative Case Series
*Corresponding Author(s):
Suresh K KarriBioplus Life Sciences Private Limited, Pharmed Gardens, Whitefield, Bangalore, Karnataka, India
Tel:+91 9739798272,
Email:sureshcology@gmail.com
Abstract
Objective of the present study was to investigate the effect of chromium polynicotinate supplementation on the visual acuity and macular thickness of the diabetic macular edema in patients with non-proliferative diabetic retinopathy through a prospective, interventional comparative case series study, was performed in 120 patients. Patients received the supplementation with or without chromium for 4 months and were followed for six months. Best-Corrected Visual Acuity (BCVA), Central Foveal Thickness (CFT), HbA1c, and the frequency of Intravitreal Bevacizumab (IVB) injection were compared. From 120 eligible patients, 90 patients involved in this study and completed the six months’ follow-up period. 51 of them were in chromium, and 39 were in the control group. The visual acuity was improved significantly from baseline in all follow-up points in both groups (P < 0.05 for all visits compared to baseline), but there was no significant difference between groups. The linear mixed model analysis showed that the mean CFT reduction was not significantly different between both groups in four follow-up visits (P=0.433, P=0.398 and P=0.630, and P=0.151, respectively). HbA1C and the average number of IVB injections were significantly lower in the chromium group, (P=0.032 and P=0.001, respectively). Chromium supplementation did not affect the visual acuity and central foveal thickness of patients with non-proliferative diabetic retinopathy and macular edema but reduced the number of IVB injections and HbA1C level.
Keywords
Central foveal thickness; Chromium polynicotinate; Diabetic retinopathy; Intravitreal bevacizumab; Visual acuity
Abbreviations
BCVA: Best-Corrected Visual Acuity
CFT: Central Foveal Thickness
CMT: Central Macular Thickness
DME: Diabetic Macular Edema
DRG: Diabetic Retinopathy Grading
ETDRS: Early Treatment Diabetic Retinopathy Study
HbA1C: Glycosylated Hemoglobin
IVB: Intravitreal Bevacizumab
NPDR: Non-Proliferative Diabetic Retinopathy
OCT: Optical Coherence Tomography
VEGF: Vascular Endothelial Growth Factor
Introduction
Diabetes mellitus is a worldwide common chronic disorder with a high burden of disease. Diabetic retinopathy is a disabling microvascular complication, causes vision loss in varying degrees in patients [1]. Diabetic macular edema (DME) is a common manifestation of this vasculopathy that can cause central visual loss and markedly affects the patient’s quality-of-life. The prevalence of DME has been reported from 3.8% to 23.8% in different studies among diabetic patients [2].
Previous studies reported that in these patients, free radical scavengers, and antioxidants like vitamin C, vitamin E, blood urate [3] or chromium are lower than healthy persons. This condition can cause oxidative damages to vascular structures [4].
Recent studies show the impressive effect of chromium on antioxidant activity and improvement of glucose hemostasis in diabetic patients [5,6]. Therefore, correction of chromium deficiency in diabetic patients can diminish oxidative stress adverse effects and prevents from diabetes complications such as vascular damages [7].
Intravitreal anti-vascular endothelial growth factor (VEGF) drugs have been investigated in numerous clinical trials, and their efficacy in the treatment of DME has been approved [8]. VEGF levels are considerably up-regulated in diabetic patients with DME, showing more extensive macular leakage than patients without significant leakage [9]. Anti-VEGF drugs may affect endothelial tight junction proteins and therefore decrease vascular permeability [10].
Most of these patients need multiple anti-VEGF injections, therefore these drugs incur high individual, medical, and societal costs. By decreasing the number of injections, the economic burden on the individual patients, their families, and society can be reduced.
This study was designed to assess the effect of oral supplements containing chromium on patients with DME who had undergone IVB injections to investigate kinetics of central macular thickness reduction, BCVA improvement and also the number of injections.
Materials and Methods
This prospective, single-center, interventional comparative case series was approved by the Institutional Review Board/Ethics Committee of Farabi Eye hospital, Iran. The study protocol and probable safety and efficacy of the interventions were explained to all participants prior to enrolment. Informed consent was obtained from all patients in writing. The protocol was executed in its approved form and there were no protocol amendments during the course of the study period. Eligible cases were the eyes with unilateral or bilateral mild to moderate non-proliferative diabetic retinopathy and clinically significant DME based on ETDRS criteria and central foveal thickness (CFT) > 320 microns. Diabetic retinopathy grading (DRG) was recorded according to the ETDRS definition for NPDR.
Exclusion criteria were a history of any treatment for DME (focal laser photocoagulation and IVB or IVT), panretinal photocoagulation, patients with proliferative diabetic retinopathy, any history of ocular surgery, glaucoma or ocular hypertension, monocularity, pregnancy, visual acuity less than or equal to 20/320 or visual acuity more than or equal to 20/32, significant media opacity and CFT <320. We also excluded patients with poor metabolic control, significant hepatic dysfunction, defined as any underlying chronic liver disease or liver function tests greater than 1.5 times the upper limit of normal or renal dysfunction, defined as serum creatinine greater than 1.5 mg/dL in baseline and follow up visits.
The enrolled DME patients received with and without chromium supplementation who were on intravitreal bevacizumab (IVB) injection. A complete ophthalmic examination including best-corrected visual acuity (BCVA) with a Snellen chart, Goldmann applanation tonometry, dilated indirect slit-lamp ophthalmoscopy, optical coherence tomography (Spectralis® Heidelberg Engineering, Heidelberg, Germany) was conducted at baseline and each follow-up at 1,2, 3 and six months after the first injection. The mean thickness on the 1-mm circle centered on the fovea was defined as central macular thickness (CMT). Complete blood count, liver and renal function test were measured at baseline, month 3, and 6 follow up visits. Serum level of Hemoglobin A1C was also measured at baseline and last visits.
All enrolled eyes received Farabi hospital standard treatment for CSME that contain 3 consecutive monthly intravitreal bevacizumab (IVB) 0.05ml (1.25 mg) (Avastin; Genen-tech, Inc., South San Francisco, CA [made for F. Hoffmann-La Roche, Ltd., Basel, Switzerland]-off label drug) injection as the loading dosage and then receiving monthly injection based on pro re-nata protocol (PRN). Retreatment indications consisted of centrally involved macular edema (more than 320 microns) based on optical coherence tomography (OCT) findings if VA was worse than 20/32. Retreatment was not performed if little or no edema involving the center of the macula or VA was equal or more than 20/32 or any clinically significant adverse effect from prior treatment was reported.
The injections were done under topical anesthesia using a 29-gauge needle in the operation room. In bilateral cases injection of both eyes was performed on the same day with separate surgical sets for each eye. All the patients were examined on days 1 and seven after the injection for anterior chamber reaction and intraocular pressure measurement.
The patients were assigned to receive either Optivision capsule (Natures Only Inc., CA, USA) that contained vitamin C 75 mg, Vitamin E 12.5 mg, Lutein 10 mg (equivalent to 300 mcg of zeaxanthin), Zinc oxide equivalent to Zinc 7.5 mg, Chromium polynicotinate equivalent to Chromium 50 mcg, Sodium Selenite equivalent to Selenium 35 mcg, and Vitamin A 5000 IU or another supplementation capsule that contained all the mentioned above minerals except chromium for at least four months after baseline injection. Follow-up visits were conducted in months one, two, three, and six after the first injection. The use of the medication and other drugs, as well as the condition of the metabolic control and patient’s daily diet, were checked in each visit.The primary outcome measure was CFT changes based on OCT at months 1, 2, 3 and 6 in comparison to baseline in each group. Secondary outcomes were BCVA change as well as the number of injections and glycaemic control.
Statistics
To describe data, we used mean, standard deviation, median, range, frequency, and percentage. To evaluate the difference between the two groups at baseline, we used Chi-Square, Fisher exact test, Mann-Whitney, and t-test. A linear multilevel model was used (with three levels: measurement in each follow-up, follow up times within eyes, and eyes within subjects to assess the effect of drug type on the changes of the outcome during the study follow-ups and to evaluate the changes in each group simultaneously. The first objective obtained by the inclusion of the interaction of time and drug. All statistical analysis performed by SPSS software (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.). A p-value of less than 0.05 was considered as statistically significant.
Results
From 120 eligible patients, a total of 180 eyes from 90 patients involved in this study and completed the six months’ follow-up period. One hundred and eighteen eyes from 59 patients were entered in the chromium group, and 62 eyes of 31 patients in the control group. The age of the patients was 38 to 78 years. Unlike Chromium group, the males were predominant in the control group (54.8%), but it was not statistically significant (P=0.468). The baseline clinical characteristics of all the patients were shown in Table 1. There was not any significant difference between the groups based on baseline age, HbA1C status, BCVA, and central foveal thickness (CFT).
|
Chromium |
Control |
P value |
|
|
Age, years |
62.37 ± 8.20 |
62.03 ± 7.14 |
0.783 |
|
Male, n, % |
58 (49.2%) |
34 (54.8%) |
0.468 |
|
HbA1C, % |
7.40 ± 1.15 |
7.84 ± 0.94 |
0.250 |
|
logMARbase |
0.61 ± 0.22 |
0.59 ± 0.13 |
0.412 |
|
CFTbase ,μm |
483.01 ± 143.46 |
548.62 ± 137.01 |
0.076 |
Table 1: The baseline clinical characteristics of the patients.
Figure 1 shows the logMAR visual acuity changes in both groups during the follow-up visits. The baseline logMAR visual acuity for chromium group was 0.61 ± 0.22 that reached to 0.52 ± 0.20, 0.56 ± 0.51, 0.56 ± 0.54, 0.56 ± 0.24 at first month, second month, third month, and six month visits, respectively. Visual acuity was improved significantly from baseline in all follow-up points in both groups (P < 0.05 for all visits compared to baseline). In the control group average logMAR visual acuity significantly increased from 0.59 ± 0.13 at baseline visit to 0.49 ± 0.15 (P < 0.001), 0.44 ± 0.15 (P < 0.001), 0.50 ± 0.14 (P < 0.001), 0.53 ± 0.14 (P < 0.001), respectively for each four follow up visits. We run a linear mixed model analysis to evaluate the effect of chromium on the logMAR visual acuity. There was no significant difference based on the mean logMAR change in any follow-up visit compared to the baseline between both groups. (P=0.589, 0.089, 0.490 and 0.493 respectively for logMAR change for each follow-up visit compared to baseline between chromium and control groups) (Figure 1). We repeated analysis after adjustment for HbA1c, levels, and IVB injection count, but no significant difference was observed. (P=0.757, 0.498, 0.292 and 0.525, respectively for four follow up visits.)
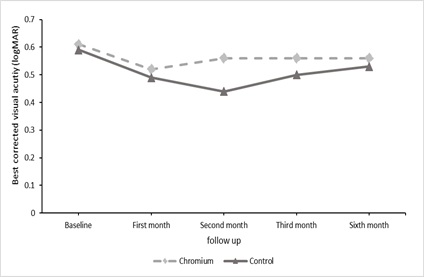 Figure 1: The difference of the logMAR visual acuity during the four follow up visits. In both groups, there was a significant change for the logMAR visual acuity at each follow-up visit compared with the baseline (P < 0.001 for all follow-up visits).
Figure 1: The difference of the logMAR visual acuity during the four follow up visits. In both groups, there was a significant change for the logMAR visual acuity at each follow-up visit compared with the baseline (P < 0.001 for all follow-up visits).
The average baseline CFT was 483.01 ± 143.46 μm for Chromium group which significantly decreased to 374.13 ± 89.43 μm (first month, P<0.001), 347.70 ± 60.68 μm (second month, P < 0.001), 337.59 ± 45.59 μm (third month, P < 0.001), 323.42 ± 38.52 μm (sixth month, P < 0.001). As well as, for control group mean CFT had a significant reduction in each follow-up visit compared to baseline and reached from 548.62 ± 137.01 μm to 423.54 ± 95.57 μm, 394.73 ± 75.64 μm, 367.88 ± 67.20 μm and 359.17 ± 58.66 μm, respectively. (P < 0.001 for all follow-up visits) (Figure 2). The mixed model analysis showed that the mean CFT reduction was not significantly different between both groups in four follow-up visits (P=0.433, P=0.398 and P=0.630, and P=151, respectively).
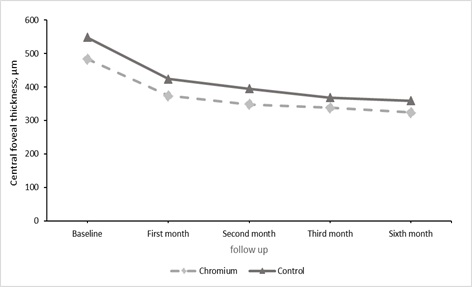 Figure 2: Mean CFT trend in follow up visit showed a significant decrease compared to baseline in both Chromium and control group
Figure 2: Mean CFT trend in follow up visit showed a significant decrease compared to baseline in both Chromium and control group
The average number of intravitreal bevacizumab injections were 4.51 ± 1.69 for the chromium and 5.44 ± 1.46 for the control group, which this difference was significant (P= 0.001).
Average HbA1C was 7.40 ± 1.15 in chromium groups and 7.84 ± 0.94 in control group that decreased significantly to 6.02 ± 1.01 and 6.88 ± 0.95, respectively. After adjustment to the baseline HbA1C level, the amount of decrease in the chromium group was significantly more than the control group (P=0.032). We also adjusted the amount of decrease for IVB injection counts.The reduction in the chromium group was still significantly higher than the control group (P=0.046).
Discussion
Microaneurysms, are tiny bulges that protrude from the vessel walls, leak or ooze fluid and blood into the retina during endothelial dysfunction. This fluid can cause swelling (edema) in the central part of the retina (macula). This is seen in serious eye complications like diabetic macular edema that can cause vision problems or blindness. Prior human studies have suggested that chromium picolinate decreases insulin levels and improves blood sugar metabolism in both obese people and people with type 2 diabetes. Essentially all the studies using chromium picolinate supplementation for impaired glucose intolerance and diabetes showed a positive effect. Although neither central foveal thickness nor LogMAR visual acuity improved in the chromium group, better glucose control has been revealed in parallel with previous studies [11-14] that may effect on anatomical and functional status in the long term. In this study, we have shown that adding chromium-containing drugs to the daily regimen in the diabetic patient with DME may lead to lower intravitreal anti-VEGF injections.
To the best of our knowledge, this is the first study that assessed the chromium effect on the diabetic retinopathy in humans. In this study, we examined the structural and visual effect of supplementing with and without chromium on a total of 180 eyes with non-proliferative diabetic retinopathy and macular edema. We also evaluated the effect of chromium on the burden of DME treatment.
Ulas et al. evaluated the effect of chromium on the diabetic retinopathy in the Long Evans rats. They used chromium histidine (CrHis) with 110 μg/kg/day dosage, and they measured systemic diabetes biomarkers and retina malondialdehyde, a retina stress indicator, and finally then run a pathological assessment of retina in diabetic and normal rats. They found that CrHis has a significant anti-stress and antidiabetic effect in diabetic retinopathy in rats. However, it is crucial to expand objective findings in an animal study to subjective visual variable in the field of diabetic retinopathy [15].
In another well-designed animal study, chromium picolinate supplementation with and without biotin plays a critical anti-diabetic role that authors suggested that the effect mediated by PPAR-γ, IRS-1, and NF-κB proteins [16]. Besides, in human studies, there are many findings in favor of the systemic anti-diabetic effects of chromium.
Huang et al. run a pooled analysis of randomized controlled trials to assess the outcomes of supplementing with chromium on the relevant metabolic biomarkers, and they found chromium decreased levels of fasting plasma glucose, hemoglobin A1c, and triglycerides. Chromium also had a positive effect on the levels of high-density lipoprotein cholesterol [17].
The mechanism of chromium function on glucose metabolism is not clearly understood. Some studies revealed that chromium increases insulin sensitivity, insulin binding ability, and upregulated insulin receptors [18,19]. Also, it enhances the response of pancreatic beta cells, tyrosine kinase activity that is the critical modulator of insulin metabolism [20]. In this study, a similar finding was made with these studies, and HbA1C reduction in chromium group was significantly higher than the control group. However, a large volume of studies showed that chromium does not have a significant effect on diabetes control. A meta-analysis was performed including 14 RCTs and a total of 840 patients with type 2 diabetes. They evaluated the supplementing with different chromium compounds on FPG and HbA1C levels on other glycemic control factors. In contrast to our result, they concluded chromium yeast, brewer’s yeast, and chromium picolinate did not display statistically meaningful impacts on HbA1C compared to placebo [21].
Another meta-analysis was performed by Suksomboon et al., and they similarly found overall, supplementing with chromium alone or with a combination of other trace elements significantly improved glycemic indexes. As well as, they reported that chromium therapy is safe and did not contain more risk than placebo [5].
In another study in the Chinese population, the serum Cr levels were decreased among patients with impaired fasting glucose, impaired glucose tolerance, type 1 diabetes and type 2 diabetes. This decrease also was observed in complicated patients with diabetic retinopathy, diabetic peripheral neuropathy, and diabetic nephropathy (P < 0.05) [22].
Unlike the above, several studies do not support the role of chromium. A randomized, double-blind clinical trial that evaluated the 400 μg/day chromium effects on the moderately-controlled diabetic patients did not show a significant A1C difference between intervention and placebo group [23]. In another clinical trial on 56 type 2 diabetic patients, chromium nicotinate 50 μg and 200 μg supplementations were used for three months. Results showed that both doses did not improve glycemic control indexes like insulin sensitivity, FPG, HbA1C, and lipid profile of individuals with diabetes [24].
Vincent suggested that the chromium may not be assumed as an essential element because it has not a proven biochemical role, and its deficiency does not cause death or failure [25]. The European Food Safety Authority Scientific accepted this opinion, and they concluded that no appropriate evidence supports supplementing with chromium in a healthy person [26].
FDA concluded that reliable evidence does not exist to assist qualified health applications for chromium picolinate and reduced risk of high blood sugar associated cardiovascular disease, retinopathy, and nephropathy when provoked by high serum glucose levels [27].
In this study, in six months, the number of IVB injections in the chromium group was about one injection less than the control group. Given the price of $ 70 per revolution in Iran, this decrease is important.
We applied Optivision Capsules that contain 50 mcg of chromium polynicotinate. Chromium has about 0.4% absorption if it exists more than 50μg in daily dietary. On the other hand, its absorption related to other factors like its formulation [28]. For example, in an equal daily intake, the chromium picolinate absorption can be up to 7 times the CrCl3; thus, bioavailability is an impactful factor [12]. In our study, the blood chromium level and its initial level were not measured. Also, the chromium absorption in the product was not precisely determined because the ineffectiveness of chromium on retinopathy factors can be due to its level, which is a limitation factor.
This study had some limitation; the sample size of the study was small. Our research runs on the patient with non-proliferative diabetic retinopathy that might restrict the generalization of outcomes on all diabetic patients with retinopathy. In terms of the effectiveness of Chromium on systemic control, we check the baseline and 24 weeks HbA1C levels that not enough to get a definite issue, so systemic deduction needs more factors and more checkpoints to evaluation. We assessed the visual effect of the Chromium supplementation with BCVA and CFT that are the variables that translate to the visual function as well, but other variables like microvascular density variables could establish for primary effects on the visual system. We recommend a largedouble blinded, randomized clinical trial with extended follow up to establish the results.
This study is an interventional comparative case series to assess the impact of Chromium supplementation on the visual acuity and central foveal thickness of patients with non-proliferative diabetic retinopathy and macular edema which did not affect both but reduced the number of IVB injection and HbA1C level.
Conflicts of Interest
The authors report no conflicts of interest.
Acknowledgement
The authors acknowledge the efforts of all the investigators and staff of the hospital.
References
- Silva JAD, Souza ECFD, Böschemeier AGE, Costa CCMD, Bezerra HS, et al. (2008) Diagnosis of diabetes mellitus and living with a chronic condition: participatory study. BMC public health 18: 699-699.
- Lee R, Wong TY, Sabanayagam C (2015) Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye and vision 2: 17-17.
- Maxwell SR, Thomason H, Sandler D, Leguen C, Baxter MA, et al. (1997) Antioxidant status in patients with uncomplicated insulin-dependent and non-insulin-dependent diabetes mellitus. Eur J Clin Invest 27: 484-490.
- Oberley LW (1988) Free radicals and diabetes. Free Radic Biol Med 5: 113-124.
- Suksomboon N, Poolsup N, Yuwanakorn A (2014) Systematic review and meta-analysis of the efficacy and safety of chromium supplementation in diabetes. Journal of Clinical Pharmacy and Therapeutics 39: 292-306.
- Yeh GY, Eisenberg DM, Kaptchuk TJ, Phillips RS (2003) Systematic Review of Herbs and Dietary Supplements for Glycemic Control in Diabetes. Diabetes Care 26: 1277-1294.
- Anderson RA, Roussel A-M, Zouari N, Mahjoub S, Matheau J-M, et al. (2001) Potential Antioxidant Effects of Zinc and Chromium Supplementation in People with Type 2 Diabetes Mellitus. Journal of the American College of Nutrition 20: 212-218.
- Wang W, Lo ACY (2018) Diabetic Retinopathy: Pathophysiology and Treatments. International journal of molecular sciences 19: 1816-1820.
- Homme RP, Singh M, Majumder A, George AK, Nair K, et al. (2018) Remodeling of Retinal Architecture in Diabetic Retinopathy: Disruption of Ocular Physiology and Visual Functions by Inflammatory Gene Products and Pyroptosis. Frontiers in physiology 9: 1268-1268.
- Behar-Cohen F, Dernigoghossian M, Andrieu-Soler C, Levy R, Cohen R, et al. (2019) Potential antiedematous effects of intravitreous anti-VEGF, unrelated to VEGF neutralization. Drug Discovery Today 24: 1436-1439.
- Ghosh D, Bhattacharya B, Mukherjee B, Manna B, Sinha M, et al. (2012) Role of chromium supplementation in Indians with type 2 diabetes mellitus. J Nutr Biochem 13: 690-697.
- Cefalu WT, Hu FB (2004) Role of Chromium in Human Health and in Diabetes. Diabetes Care 27: 2741-2751.
- Anderson RA, Cheng N, Bryden NA, Polansky MM, Cheng N, et al. (1997) Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes 46: 1786-1791.
- Anderson RA (1998) Chromium, Glucose Intolerance and Diabetes. Journal of the American College of Nutrition 17:548-555.
- Ulas M, Orhan C, Tuzcu M, Ozercan IH, Ozercan N, et al. (2015) Anti-diabetic potential of chromium histidinate in diabetic retinopathy rats. BMC Complement Altern Med 15: 537-543.
- Sahin K, Tuzcu M, Orhan C, Sahin N, Kucuk O, et al. (2013) Anti-diabetic activity of chromium picolinate and biotin in rats with type 2 diabetes induced by high-fat diet and streptozotocin. British Journal of Nutrition 110: 197-205.
- Huang H, Chen G, Dong Y, Zhu Y, Chen H (2018) Chromium supplementation for adjuvant treatment of type 2 diabetes mellitus: Results from a pooled analysis. Mol Nutr Food Res 62: 438-441.
- Penumathsa SV, Thirunavukkarasu M, Samuel SM, Zhan L, Maulik G, et al. (2009) Niacin bound chromium treatment induces myocardial Glut-4 translocation and caveolar interaction via Akt, AMPK and eNOS phosphorylation in streptozotocin induced diabetic rats after ischemia-reperfusion injury. Biochim Biophys Acta 1792: 39-48.
- Panchal SK, Wanyonyi S, Brown L (2017) Selenium, Vanadium, and Chromium as Micronutrients to Improve Metabolic Syndrome. Current Hypertension Reports 19: 10-14.
- Lewicki S, Zdanowski R, Krzyzowska M, Lewicka A, Debski B, et al. (2014) The role of Chromium III in the organism and its possible use in diabetes and obesity treatment. Ann Agric Environ Med 21: 331-335.
- Yin RV, Phung OJ (2015) Effect of chromium supplementation on glycated hemoglobin and fasting plasma glucose in patients with diabetes mellitus. Nutr J 14:891-894.
- Zhou Q, Guo W, Jia Y, Xu J (2019) Comparison of Chromium and Iron Distribution in Serum and Urine among Healthy People and Prediabetes and Diabetes Patients. Biomed Res Int 3: 801-809.
- Kleefstra N, Houweling ST, Bakker SJL, Verhoeven S, Gans ROB, et al. (2007) Chromium Treatment Has No Effect in Patients With Type 2 Diabetes in a Western Population. A randomized, double-blind, placebo-controlled trial 30: 1092-1096.
- Guimaraes MM, Martins Silva Carvalho AC, Silva MS (2013) Chromium nicotinate has no effect on insulin sensitivity, glycemic control, and lipid profile in subjects with type 2 diabetes. J Am Coll Nutr 32: 243-250.
- Vincent JB (2017) New Evidence against Chromium as an Essential Trace Element. The Journal of Nutrition 47: 2212-2219.
- EFSA Panel on Dietetic Products, NDA (2014) Scientific Opinion on Dietary Reference Values for chromium. EFSA Journal 12: 3845.
- Qualified Health Claims: Letter of Enforcement Discretion (2017) Chromium Picolinate and Insulin Resistance 18: 37-39.
- Broadhurst CL, Domenico P (2006) Clinical Studies on Chromium Picolinate Supplementation in Diabetes Mellitus—A Review. Diabetes Technology & Therapeutics 8: 677-687.
Citation: Mirshahi A, Ghahvehchian H, Riazi-Esfahani H, Ghods S, Bazvand F, et al. (2021) The Effect of Chromium in the Management of Diabetic Macular Edema: An Interventional Comparative Case Series. J Ophthalmic Clin Res 8: 083.
Copyright: © 2021 Ahmad Mirshahi, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

