
The Effect of Sandy Surfaces on Touch DNA
*Corresponding Author(s):
Salem Alketbi KGeneral Department Of Forensic Science And Criminology, Dubai Police, United Arab Emirates
Tel:00447774141205,
Email:alkitbe.11@hotmail.com
Abstract
Touch DNA profiling is an important tool to solve the mystery of many cases, especially when other biological evidences cannot be found in crime scene. However, there are many variables that influence Touch DNA profiling such as recovery techniques and extraction. In addition, effect of environmental factors on items found outdoor such as sand can impact on the process. Therefore the aim of this experiment was to test how sandy surfaces can affect the recovery of Touch DNA Profiling by validation two recovery methods and two extraction kits that are widely used in the DNA forensic field.
Keywords
DNA recovery; Forensic DNA; PrepFiler Express BTA™; QIAamp® DNA Investigator; Quantifiler™ Human DNA Quantification Kit; Touch DNA
INTRODUCTION
Touch DNA profiling is an important tool to solve the mystery of many cases, especially when other biological evidences cannot be found in crime scene. However, there are many variables that influence Touch DNA profiling such as recovery techniques and extraction [1-3]. In addition, heat and humidity on items found outdoor can reduce or loss of trace DNA [4,5]. Another issue with environmental factors that can influence Touch DNA recovery on items found outdoor is dust or sand, especially in hot climates such as Dubai where sands move all the time because of the winds.
Therefore the aim of this experiment was to test how sandy surfaces can affect the recovery of Touch DNA Profiling by validation two recovery methods and two extraction kits that are widely used in the DNA forensic field.
MATERIALS AND METHODS
Experimental set up and deposition
A selection of four surfaces (stainless steel; smooth non-porous, glass; smooth non-porous, textured wood; rough porous and textured plastic; rough non-porous) were chosen to replicate common items encountered in crime scenes and to have a variety of surfaces. All non-porous surfaces were sterilised by 2% virkon and ultraviolet radiation (UV) for 15 min, and only textured wood was irradiated with UV for 25 min.
For DNA deposition, a participant was asked to wash his hands with antibacterial soap and refrain from undertaking any activity for 10 minutes. Then, charge the fingers of both hands with eccrine sweat by touching behind their ears or forehead to load them with enough DNA. The participant was then asked to touch the surfaces using their index, middle, and ring fingers of both hands separately for deposition by applying medium pressure on 5 x 7 cm area of the surface for 1 minute. The same procedure was repeated on all the surfaces for equal deposition on each surface.
After deposition of DNA, sand from Dubai (common sand found outdoors) was left on the surfaces, which were then placed in High temperature with moderate humidity (40°C/50%) for three hours to simulate Dubai weather (n=48 – three replicates for each variable).
DNA recovery and extraction
Two methods were used to recover the touch DNA, Copan cotton swab (150C) (CS) and Copan nylon flocked swab (4N6 FLOQSwabs®) (NS). Before collection, 100μL of sterile distilled water was applied to moisten CS using a plastic spray bottle technique (developed in Dubai police forensic DNA lab; each single spray contains approximately 50μL). For NS, 30μL of sterile distilled water was applied to moisten the swab using a pipette as recommended by the manufacturer.
Full swabs head were extracted by PrepFiler Express BTA™ kit (Thermo Fisher Scientific) (EX1) using an AutoMate Express Forensic DNA Extraction System according to the manufacturers’ recommendations and manually using the QIAamp® DNA Investigator Kit (Qiagen) (EX2) as per the manufacturers’ protocol. However, with EX2 nylon swabs were extracted using NAOBasket™ as recommended by Copan to increase the DNA yield.
DNA quantification, amplification and analysis
Extracted samples were quantified using the Quantifiler® Human DNA Quantification Kit, Quant Studio 5 Real-Time PCR (qPCR) and HID Real-Time PCR analysis software v1.3 according to the manufacturer’s instructions (Thermo Fisher Scientific). Amplification was performed using the Global Filer™ PCR Amplification Kit (Thermo Fisher Scientific) according to the manufacturer’s recommendation, following 30 cycles protocol.
Then the data were analysed using GeneMapper® ID-X Software Version 1.2 (Thermo Fisher Scientific). Statistical analysis on the tested variables was performed with RStudio using factorial analysis of variance (ANOVA). Blanks were taken from surfaces after sterilization, and negative controls for the collection and extraction methods, all of which were negative for DNA when quantified.
RESULTS AND DISCUSSION
The amount of DNA collected from the sandy surfaces was significantly affected by collection method (F1,32 = 7557.47, p < 0.05), extraction type (F1,32 = 817.26, p < 0.05) and the interaction between collection and extraction (F1,32 = 172.22, p < 0.05) (Figure 1).
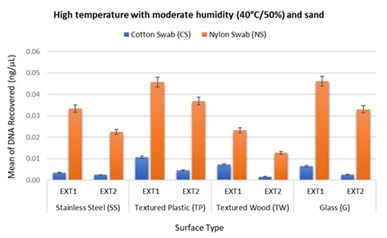
Figure 1: Amount of DNA recovered from four sandy surfaces (n = 48) using cotton and nylon swabs, then extracted by PrepFiler Express BTA™ kit (EX1) and QIAamp® DNA Investigator Kit (EX2). Error bars represent 95% confidence intervals.
Samples performed better when extracted by PrepFiler Express BTA™ kit (EX1) than the QIAamp® DNA Investigator Kit (EX2), when the collected swabs contained sand. Nevertheless, the Nylon Swab was the best performer of collection touch DNA from the sandy surfaces, when compared to cotton swabs (Figure 2). When using both swabs to collect DNA from the sandy surfaces, the cotton swab collected much more sand than the nylon swab. That can be caused by the amount of distilled water used with the swabs (100μL with cotton swab and 30μL with nylon swab), or the fact that the cotton swab retains much more sand.
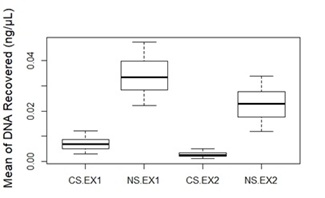
Figure 2: The mean of DNA recovered from the sandy surfaces (n=48) using cotton (CS) and nylon swabs (NS), then extracted by PrepFiler Express BTA™ kit (EX1) and QIAamp® DNA Investigator Kit (EX2). Error bars represent standard Error.
Samples collected from stainless steel were amplified to validate the quality of samples collected. Samples collected by nylon swab produced full profiles, and samples collected by cotton swab produced almost full profiles with few allele dropouts (Figure 3).
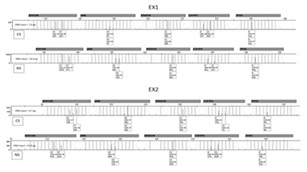
Figure 3: Samples collected from nylon swab and Cotton swab.
CONCLUSION
Sand on the surfaces found outdoors can influence the amount of the DNA recovered from touched items. A nylon swab is an advisable to use as a collection method from items found outdoors in sandy environments such as Dubai, in combination with the PrepFiler Express BTA™ extraction kit.
CONFLICT OF INTEREST
None.
ACKNOWLEDGEMENTS
This study was approved by General Department of Forensic Science and Criminology in Dubai Police and Ethical approval was granted by School of Forensic and Applied Sciences, and the University of Central Lancashire’s Research Ethics Committee (ref. no. STEMH 912). Many thanks to COPAN DIAGNOSTICS INC. for supporting this experiment with free swabs, and to Thermo Fisher Scientific™ for the discounts on their products.
REFERENCES
- Alketbi SK (2018) The Affecting Factors of Touch DNA. J Forensic Res 9: 424.
- Verdon TJ, Mitchell RJ, Oorschot RA (2014) Swabs as DNA collection devices for sampling different biological materials from different substrates. J Forensic Sci 59: 1080-1089.
- Ip SC, Lin SW, Lai KM (2015) An evaluation of the performance of five extraction methods: chelex® 100, QIAamp® DNA blood mini kit, QIAamp® DNA investigator kit, QIAsymphony® DNA Investigator® kit and DNA IQ™. Sci Justice 55: 200-208.
- Raymond JJ, Walsh SJ, Van Oorschot RA, Gunn PR, Evans L, et al. (2008) Assessing trace DNA evidence from a residential burglary: abundance, transfer and persistence. Forensic Science International: Genetics Supplement Series 1: 442-443.
- Poinar HN (2003) The top 10 list: criteria of authenticity for DNA from ancient and forensic samples. In International congress series 1239: 575-579.
Citation: Alketbi SK, Goodwin W (2019) The effect of sandy surfaces on Touch DNA. Forensic Leg Investig Sci 5: 034.
Copyright: © 2019 Salem Alketbi K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

