
The Oleaginous Ossein - Chondroid Lipoma
*Corresponding Author(s):
Anubha BajajConsultant Histopathology, A.B. Diagnostics, New Delhi, India
Email:anubha.bajaj1@outlook.com
INTRODUCTION
Chondroid lipoma is an exceptional, benign neoplasm composed of mature adipocytes, lipoblasts and intermingled myxochondroid stroma. Chondroid lipoma predominantly appears within third decade of life wherein a female preponderance is observed. The neoplasm is predominantly situated within subcutaneous adipose tissue, submucosa, superficial muscular fasciaor as intramuscular lesion within skeletal muscles of proximal extremities, limb girdles, trunk or head and neck. Micro-section displays a tumefaction composed of fascicles, nests and sheets of eosinophilic, multi-vacuolated cells variably admixed with mature adipocytes. The cellular component is enmeshed within a myxoid and chondroid-like stroma. Multi- vacuolated cells, akin to chondroblasts or cells of hibernoma, are imbued with adipose tissue and glycogen, as detected by Oil-red O and Periodic Acid Schiff’s (PAS) stains respectively. Chondroid lipoma is immune reactive to S100 protein andvimentin. Tumefaction depicts chromosomal translocation t(11;16), (q13;p13) besides C11 or f95-MKL2 fusion genes. Chondroid lipoma requires a demarcation from lipoma, neurofibroma, schwannoma, teratoma, myxoid chondrosarcoma or extra-skeletal chondrosarcoma. Magnetic Resonance Imaging (MRI) is beneficial in diagnosing chondroid lipoma. Surgical eradication of the neoplasm is generally curative.
PREFACE
Lipomatous mesenchymal neoplasia are a common variety of soft tissue tumours wherein conventional lipoma is frequent. Albeit, spindle cell lipoma, pleomorphic lipoma or lipoblastoma can be misinterpreted as liposarcoma.Chondroid lipoma is designated as an exceptional, benign neoplasm composed of mature adipocytes, lipoblasts and intermingled myxochondroid stroma, initially scripted by Meis and Enzinger in 1993 [1]. Chondroid lipoma as a lipomatous neoplasm recapitulates pseudo-sarcoma like histology. Unique cellular population of chondroid lipoma denominates embryonal adipose tissue intermixed with embryonal cartilage, thus signifying a dual tissue concurrence. The neoplastic adipose tissue derivative depicts distinct foci of chondro- osseous metaplasia. Heterologous interstitial cells are absent [1,2]. A cellular neoplasm of distinctive morphology, chondroid lipoma can be misdiagnosed as round cell liposarcoma or chondrosarcoma. With misinterpretation as a malignant neoplasm, irrelevant lymph node dissection, extensive tumour resection or amputation of incriminated areas can ensue. Cogent diagnosis and appropriate therapy of chondroid lipoma essentially lacks consensus [1,2].
DISEASE CHARACTERISTICS
Chondroid lipoma predominantly appears within third decade of life. A female preponderance is observed as an estimated 80% instances are delineated in females with a female to male proportion of 4: 1.Female preponderance implies the incrimination of hormonal factors in disease pathogenesis. Median age of disease emergence is 36 years wherein the neoplasm is discovered betwixt 14 years to 70 years. An obvious geographical or ethnic trend of disease occurrence is absent [2,3]. Neoplastic discernment can occur within 6 days to 10 years. Chondroid lipoma appears within the trunk (26.14%), lower limbs (36.4%), upper limbs (13.64%), oral cavity (7.95%), feet (4.55%) or uterus (1.14%). Nomenclature of chondroid lipoma is indicative of pertinent histological pattern as the neoplasm distinctively comprises of mature adipose tissue cells, lipoblasts, cartilaginous chondroblasts and hyaline matrix. Stromal mucins detected within chondroid lipoma are indicative of chondroid differentiation [2,3].
CLINICAL ELUCIDATION
Chondroid lipoma is predominantly situated within subcutaneous adipose tissue, submucosa, superficial muscular fascia or as intramuscular lesion within skeletal muscles of proximal extremities, limb girdles, trunk or head and neck. Chondroid lipoma is infrequently discerned within oral cavity or myometrial leiomyomata. The neoplasm represents as a painless, gradually progressive, well defined, non tender, subcutaneous nodule [2,3]. Chondroid lipoma situated within trunks or limbs manifest as a painless nodule, although tenderness, tingling and radiating pain can concur. Painful lesions necessitate distinction from schwannoma or neurofibromatosis. Oral chondroid lipoma represents as soft tissue polyps whereas intrauterine neoplasms can engender unascertained vaginal bleeding. Chondroid lipoma is preponderantly composed of white, mature adipocytes with an absence of true cartilaginous differentiation [3].
HISTOLOGICAL ELUCIDATION
Chondroid lipoma appears as solid, firm, yellowish or reddish- brown nodule of variable magnitude with definitive tumour margins. Cut surface is yellow- brown, pearly, grey/white or reddish- brown with a gelatinous texture and occasional haemorrhagic foci. On gross examination, a well encapsulated, lobulated, yellowish or whitish, partially skin covered neoplasm of 4 centimetre median magnitude is discerned although tumour dimension can extend up to 11 centimetres. The elliptical or spheroidal tumefaction is firm, mobile, non-fluctuant, depicts a non-breached extraneous capsule and lacks adherence to superimposed cutaneous surface or adjacent soft tissue [3,4]. Upon capsular incision, lobular, yellowish or reddish- brown tumefaction is discerned which displays miniature foci of chalky white material. Frozen section delineates enlarged quantities of mature cartilage, foci of ossification and proliferating peripheral compartment composed of fibrous tissue and mature adipose tissue. Cellular atypia is absent. Ultrasound guided fine needle aspiration demonstrates accumulation of adipose tissue with focal differentiation and maturation of clusters of adipocytes of diverse magnitude. Mature lipocytes or lipoblast- like cells are disseminated within a chondromyxoid matrix. Adipoblasts are quantifiably detected along with foci of mature cartilage and bone. However, segregation from an atypical lipoma with foci of mature cartilage and ossification can be challenging [3,4]. Chondroidlipoma is a well circumscribed neoplasm constituted by mature adipocytes, chondroidtissue denominated by vacuolated, lipoblast-like or chondroblastic cells and an encompassing myxo-hyaline matrix. Micro-section displaysa tumefaction composed of fascicles, nests and sheets of eosinophilic, multi-vacuolated cells variably admixed with mature adipocytes. The cellular component is enmeshed within a myxoid and chondroid-like stroma [3,4]. Well defined, tumorous aggregates and cords of miniature to medium sized, multi-vacuolated cells are discerned. The cellular component simulates lipoblasts, chondroblasts or cells of hibernoma cells. Nuclear outlines of tumour cells are complex. The encompassing, prominently chondromyxoid matrix demonstrates variable quantities of mature adipose tissue and exceptionally, metaplastic bone [4,5]. Chondroid lipoma is constituted by varying proportions of mature adipocytes, adipoblasts and transparent, mucoid, cartilaginous zones with focal ossification. Enlarged, mature adipocytes with vacuolated, lipid-rich cytoplasm and compressed, eccentric nucleus are exhibited. Miniature adipoblasts configure aggregates, fascicles, nests and cord- like arrangements. Adipoblasts with eosinophilic, multi-vacuolated cytoplasm exemplify several vacuoles of varying magnitude along with miniature nuclei demonstrating minimal anisocytosis and pleomorphism. Nucleolar and intra-nuclear pseudo-inclusions are observed [4,5]. Classic histologic pattern denominates distinct cellular categories such as mature adipose tissue cells, lipoblast- like cells with vacuolated cytoplasm and centric nuclei or elliptical to spheroidal cells with eosinophilic cytoplasm. Nuclei are minimally pleomorphic although mitotic activity is absent to minimal. The encapsulated neoplasm comprised of miniature, spheroidal cells articulates loosely cohesive sheets, clusters and cords intermingled withinmyxoid, hyalinised or cartilaginous intercellular matrix. Multi- vacuolated cells, akin to chondroblasts or cells of hibernoma, are imbued with adipose tissue and glycogen, as detected by Oil-red O and Periodic Scid Schiff’s (PAS) stains respectively [3,5]. Transparent, mucoid, cartilaginous - like zones along with variable, articulated matrices are numerous. Partial deposition of inter-cellular cellulose amidst mature adipocytes and adipoblasts is discerned. Vascular configurations are denominated although a typical, plexiform, vascular morphology, as detected in myxoidliposarcoma, is absent. With variable vascularity, prominent vasculature demonstrates diverging thickness of blood vessel walls [4]. Focal fibrosis, haemorrhage and haemosiderin pigment deposits are exhibited along with eosinophilic foci of fibrinous matrix. Cellular or nuclear pleomorphism or atypia are minimal or absent. Foci of mature hyaline cartilage are absent. On ultrastructural examination, abundant intracytoplasmic lipid and glycogen molecules are observed. Numerous pinocytotic vesicles are characteristically delineated within white adipocytes. Tumour cells recapitulate embryonal fat or can minimally resemble embryonal cartilage. Knob- like protrusions arising from cell membrane, composed of granular, amorphous, fibrillary material are exemplified. Mitochondria and lysosomes are insignificant [4,5] (Figures 1-8).
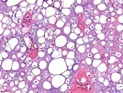 Figure 1: Chondroid lipoma depicting cords of small to medium, multi-vacuolated cells lacking cellular atypia, interspersedwithin a myxoid and chondroid matrix [1].
Figure 1: Chondroid lipoma depicting cords of small to medium, multi-vacuolated cells lacking cellular atypia, interspersedwithin a myxoid and chondroid matrix [1].
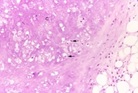 Figure 2: Chondroid lipoma delineating aggregates and nests of miniature to medium multi-vacuolated cells with uniform nuclei, lack of mitosis, foci of mature adipose tissue and an intermixed chondro-myxoid matrix [2].
Figure 2: Chondroid lipoma delineating aggregates and nests of miniature to medium multi-vacuolated cells with uniform nuclei, lack of mitosis, foci of mature adipose tissue and an intermixed chondro-myxoid matrix [2].
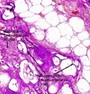 Figure 3: Chondroid lipoma demonstrating clusters of eosinophilic multi-vacuolated cells interspersed in a myxo-chondroid stroma and foci of mature adipose tissue [3].
Figure 3: Chondroid lipoma demonstrating clusters of eosinophilic multi-vacuolated cells interspersed in a myxo-chondroid stroma and foci of mature adipose tissue [3].
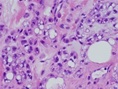 Figure 4: Chondroid lipoma exhibiting aggregates of eosinophilic, multi-vacuolated cells intermixed within a chondro-myxoid stroma and mature adipose tissue cells [4].
Figure 4: Chondroid lipoma exhibiting aggregates of eosinophilic, multi-vacuolated cells intermixed within a chondro-myxoid stroma and mature adipose tissue cells [4].
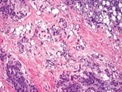 Figure 5: Chondroid lipoma composed of accumulated, eosinophilic, multi-vacuolated cells admixed within a myxoid and chondroid matrix besides foci of mature adipose tissue [5].
Figure 5: Chondroid lipoma composed of accumulated, eosinophilic, multi-vacuolated cells admixed within a myxoid and chondroid matrix besides foci of mature adipose tissue [5].
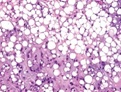 Figure 6: Chondroid lipoma constituted by clumps of eosinophilic cells with multiple vacuoles, foci of mature adipose tissue and a myxo-chondroid matrix [5].
Figure 6: Chondroid lipoma constituted by clumps of eosinophilic cells with multiple vacuoles, foci of mature adipose tissue and a myxo-chondroid matrix [5].
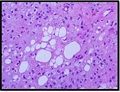 Figure 7: Chondroid lipoma comprised of small cells with eosinophilic cytoplasm, multi-vacuolated cells commingled with myxo-chondroid matrix and a lack of atypia [6].
Figure 7: Chondroid lipoma comprised of small cells with eosinophilic cytoplasm, multi-vacuolated cells commingled with myxo-chondroid matrix and a lack of atypia [6].
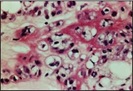 Figure 8: Chondroid lipoma depicting cellular nests with eosinophilic, multi-vacuolated cytoplasm, abundant myxoid and chondroid matrix with few mature adipocytes [7].
Figure 8: Chondroid lipoma depicting cellular nests with eosinophilic, multi-vacuolated cytoplasm, abundant myxoid and chondroid matrix with few mature adipocytes [7].
IMMUNE HISTOCHEMICAL ELUCIDATION
Miniature, vacuolated cells of chondroid lipoma are immune reactive to S100 protein where mature adipocytes are intensely immune reactive, in contrast to weakly reactive lipoblasts. As cells are immune reactive to vimentin, maturity and differentiation of adipoblasts is concurrent with immune reactivity to vimentin (93.75%) and S100 protein (93.33%). Certain tumours are variably and focally immune reactive to CD68 (64.29%) and cytokeratin (25.93%). Intracellular glycogen is detected by periodic acid Schiff’s (PAS) stain. Alcian blue and toluidine blue can highlight chondroitin substrate. Immune reactive collagen IV circumscribes tumour cell aggregates. Cyclin D1 is intensely immune reactive [5,6]. Cells are immune non reactive to Epithelial Membrane Antigen (EMA), Human Melanoma Black 45 (HMB45) antigen, Smooth Muscle Actin (SMA), Muscle Specific Actin (MSA) and Glial Fibrillary Acidic Protein (GFAP). Nuclear proliferation labelling index MIB-1 or Ki67 is below <1%. Although female preponderance is observed, the neoplasm is devoid of sex hormone related receptors such as oestrogen (ER), Progesterone (PR) or Androgen Receptors (AR) [5,6]. Cytogenetic analysis demonstrates chromosomal translocation t(11;16), a genomic alteration also exemplified in hibernoma. Chondroid lipoma frequently depicts chromosomal translocation t(11;16), (q13;p13) besides presence of C11 or f95-MKL2 fusion genes. Equilibrium chromosomal translocation of t(11;16), (q13;p12-13) is enunciated. Although lipoma and adjunctive hibernating neoplasia demonstrate chromosomal anomalies of 11q13, aberration 16p12-13 is absent, a feature enunciated in chondroid lipoma [5,6].
DIFFERENTIAL DIAGNOSIS
Chondroid lipoma can be misdiagnosed and requires a demarcation from benign conditions as lipoma, neurofibroma, schwannoma or teratoma. Chondroid lipoma mandates a segregation from malignant neoplasia as myxoid chondrosarcoma or extra-skeletal chondrosarcoma which are constituted by mature chondrocytes, adipoblasts and mature adipocytes with an intermingled myxoid matrix. However, combined genetic, histopathological and Magnetic Resonance Imaging (MRI) can assist appropriate demarcation [6]. Chondroid lipoma necessitates a segregation from Lipoma with chondroid metaplasia, a neoplasm which exemplifies foci of true cartilage. Extra-skeletal chondroma,which is a benign neoplasm principally arising within distal extremities. The neoplasm is composed of mature hyaline or true cartilage and exhibits multinucleated giant cells. Tumour cells are devoid of intracytoplasmic vacuoles as discerned with mature adipose tissue [6,7]. Mixed tumour, which delineates foci of epithelial differentiation with keratin production and an absence of lipoblasts. Extra-skeletal myxoid chondrosarcoma, which is a predominantly lobulated tumefaction traversed by prominent fibrous tissue septa and circumscription with thin, peripheral capsule. Adipocytes and lipoblasts are usually absent. Mitotic figures and foci of necrosis are frequent. Chondroblasts constituting the tumour are spheroidal and delineate minimal or absent intracytoplasmic vacuoles or foci of mature adipose tissue [6,7]. Myxoidliposarcoma, which commonly appears in diverse sites. Microscopically, it lacks prominent cords or cellular clusters and is characteristically composed of true lipoblasts and is imbued with delicate, plexiform, capillary-rich matrix. Genetic translocation t(12;16) is detected within the neoplasm. Aforesaid malignant sarcomas delineate an inferior prognostic outcome and therapeutically entail radical surgical manoeuvers with post operative radiotherapy [6,7].
INVESTIGATIVE ASSAY
Chondroid lipoma lacks characteristic features of imaging on account of inconstant proportion of intermingled adipose tissue and cartilage. On ultrasonography, a heterogeneous mass with minimal echo, uneven morphology, mildly enhanced intensity echo, irregular internal echo and blurred outline is discerned [7]. Ultrasonography of chondroid lipoma demonstrates a lobulated tumour architecture due to several fibrous tissue membranes traversing the neoplasm with consequent poor quality images. The tumefaction is comprised of abundant quantities of cartilage- like tissue, thus engendering an intense echogenicity. Often, a heterogeneous, mixed signal is delineated upon imaging due to content and variable morphology of cartilage- like foci, which preponderantly demonstrate an irregular outline [7,8]. Plain radiography of chondroid lipoma indicates the occurrence of calcific structures or ossification within the tumefaction although cartilaginous foci may not be ascertained. On Computerized Tomography (CT), a minimal density shadow with an approximate value of -80 Hounsfield units is discerned. A nodular, density shadow of soft tissue and dot- like, high density shadow are delineated [7,8]. Chondroid lipoma requires diagnostic consideration even with the emergence of non specific findings on radiography or CT. Nevertheless, as an elevated proportion of false negative diagnosis is engendered with plain radiography or CT, routine employment of aforesaid manoeuvers is not recommended [7,8]. MRI is beneficial in diagnosing chondroid lipoma. An admixture of enhanced and minimal signal intensities is detected upon T1 weighted imaging, Short-Time Inversion Recovery (STIR) sequences and fat-suppression sequence whereas enhanced signal density is observed upon T2 weighted imaging. Heterogeneous saturation of adipose tissue appears upon T2 weighted fat- saturated imaging [8,9]. Minimal signal intensity upon T1 weighted imaging and enhanced signal intensity upon T2 weighted imaging are indicative of chondroid tissue. Enhanced signal intensity upon T1 weighted imaging are prohibited on STIR imaging, thus suggesting concurrence of adipose tissue along with non uniform fat saturation upon T2 weighted fat- saturated imaging. A distinct, “fat ring” sign is discerned upon contrast- enhanced and fat- saturated imaging. The sign, indicative of cartilage tissue within tumour centric zone, depicts minimal signal intensity upon T1 weighted imaging, enhanced signal intensity upon T2 weighted imaging and a delayed ring or arc- shaped enhancement pattern following injectable contrast agent. Circumscribing adipose tissue exhibits an intense signal as compared to skeletal muscle upon T1 or T2 weighted imaging and heterogeneous fat saturation upon T2 weighted fat-saturated imaging [8,9]. Single- Photon Emission Computerized Tomography (SPECT-CT) is an investigative option beneficially adopted in separating diverse tumours. Elevated affinity for Fluorine Labelled- 18Fluoro-Deoxy-Glucose (F-FDG) is observed wherein accumulation of aforesaid molecule is concurrent with enhanced glucose metabolism within tissues and organs. Affinity for 18F-FDG is frequently discerned with malignant tumefaction or inflammatory conditions. Thus, employment of SPECT/CT with 18F-FDG is nonspecific in discerning chondroid lipoma [8,9]. SPECT-CT imaging depicts adjacent bone as a radioactive, concentrated shadow and enveloping soft tissue as an elliptical, minimally dense shadow with value of -86 Hounsfield units. Separation of various neoplastic components, irregular density of soft tissue and enhanced tissue density are also observed along with density shadow of around 166 Hounsfield units with enhanced radioactivity uptake. The tumefaction displays an affinity for Methylene-Diphosphonate (99m Tc-MDP), a molecule which is specific for bone or cartilage tissue. Therefore, application of 99m Tc-MDP is significantly superior than 18F-FDG in detecting chondroid lipoma. Also SPECT/CT is an expensive investigative modality, hence the procedure may not be advantageous in routine analysis [8,9].
THERAPEUTIC OPTIONS
Chondroid lipoma as a clinically benign neoplasm can be appropriately alleviated with localized surgical resection. Surgical eradication of the neoplasm is generally curative. Chondroid lipoma can be suitably treated with simple surgical resection in the absence of postoperative adjuvant therapy. Surgical extermination of chondroid lipoma is associated with adequate post-operative recovery and subsequent monitoring is unnecessary [10]. Diode laser therapy can be employed for eradicating tumour tissue situated in precarious zones as the oral cavity. Laser therapy is associated with lack of intraoperative haemorrhage or subsequent suturing and demonstrates minimal post-operative pain, haemorrhage, swelling or inflammation, in contrast to adjunctive techniques. Adoption of laser therapy is accompanied by rapid wound healing [10]. The essentially benign neoplasm is devoid of tumour reoccurrence, metastasis or malignant metamorphoses. Following surgical treatment, adjuvant therapy is unnecessary as extended monitoring reveals a lack of tumour reoccurrence or metastasis [10].
FIGURE REFERENCES
- Image 1 Courtesy: Webpathology
- Image 2 Courtesy: Science direct
- Image 3 Courtesy: Histopathology.india.net
- Image 4 Courtesy: Pathology outlines
- Image 5 and 6 Courtesy: Basic medical key
- Image 7 Courtesy: Journal of case reports and images
- Image 8 Courtesy: Indian Journal of Pathology and Microbiology
REFERENCES
- Meis JM, Enzinger FM (1993) Chondroid lipoma: A unique tumour simulating liposarcoma and myxoid chondrosarcoma. Am J Surg Pathol 17: 1103-1112.
- Kindblom LG, Meis-Kindblom J (1995) Chondroid lipoma-an ultrastructural and immune histochemical analysis with further observations regarding its’ differentiation. Hum Pathol 26: 706-715.
- Huang C, Guo W, Qu W, Zhu Z, Li R (2019) Characteristics of chondroid lipoma- a case report and literature review. Medicine (Baltimore) 98: e15587.
- Fournel L, Rapicetta C, Fraternali A, Bellafiore S, Paci M, et al. (2018) Fibrous dysplasia of the rib mimicking a malignant bone tumour at SPECT/CT with 99m Tc-MDP. Clin Nucl Med 43: 346-348.
- Tatsiou Z, Mavropoulou S (2017) Chondroid lipoma of the breast- a rare case report. Virchows Arch 471: 63-64.
- Ren S, Yue Y (2017) A case of atypical chondroid lipoma. Radiologic Pract 32: 776-777
- Kapse SUS, Javalgi A, Javalgi AP (2017) Chondroid lipoma in left thigh- a rare case report. J Clin Diagn Res 11: 17-18.
- Yildiz A, Aydingoz U, Sökmensüer C, Karçaaltincaba M (2015) Intramuscular chondroid lipoma- magnetic resonance imaging diagnosis by ‘fat ring’ sign. Balkan Med J 32: 107-110.
- Liu H, Long D (2015) Chondroid lipoma of the abdominal wall: one case report. J Med Imag 25: 1013-1014.
- Yaranal PJ, Hegde V (2013) Chondroid lipoma of the thigh - a case report. Indian J Pathol Microbiol 56: 464-465.
Citation: Bajaj A (2021) The Oleaginous Ossein- Chondroid Lipoma. J Brain Neursci 5: 016.
Copyright: © 2021 Anubha Bajaj, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

