
Journal of Ophthalmology & Clinical Research Category: Clinical
Type: Research Article
The Variation in Surgical Technique and Role of Physician Experience in Pterygium Surgical Outcomes
*Corresponding Author(s):
Shameema SikderWilmer Ophthalmological Institute, Johns Hopkins University School Of Medicine, Baltimore, Maryland, United States
Tel:+1 2404821100,
Email:ssikder1@jhmi.edu
Received Date: May 27, 2015
Accepted Date: Jul 22, 2015
Published Date: Aug 05, 2015
Abstract
Objectives: To evaluate surgical management of pterygium at a teaching hospital and the role of physician experience in recurrence and complication rates after pterygium surgical removal.
Methods: This 9-year retrospective study reviewed 119 primary pterygium eyes receiving pterygium excision at Wilmer eye institute (Baltimore, Maryland). The procedure data included the procedure type, adjuvants, attachment and surgeon experience (attending or trainee). Bare sclera, conjunctival autograft, amniotic membrane graft, and primary conjunctival closure techniques were analyzed for complications and recurrence.
Results: Average follow-up was 16.7 ± 22.0 months. The overall recurrence rates were: amniotic membrane 19% (n = 37), bare sclera 20% (n = 5), conjunctival autograft 16% (n = 68), and primary conjunctival closure 22% (n = 9). Statistically significant differences in recurrence rates between experience groups was only reached in the conjunctival autograft (p = 0.038, Fisher exact), but not after controlling for mitomycin C. Trainees used mitomycin C in conjunctival autograft cases more often (81% of cases versus 38% of attending cases, p = 0.001, Fisher Exact). No significant differences existed in recurrence time between groups (p = 0.164, log-rank). The average time to recurrence was 10.7 ± 12.0 months and 29% of recurrences occurred later than 12 months post-operatively. There were no statistically significant differences in complication rates.
Conclusion: A variety of techniques were utilized at an academic center and included amniotic membrane grafts, conjunctival autografts, bare sclera excision, and primary conjunctival closure. These procedures often used adjuvants like mitomycin C, or in a minority of cases used fibrin glue in place of, or in conjunction with sutures. In regards to the role of experience, trainees did not have statistically significant differences in complication rates and no significant difference in recurrence rates for any procedure after controlling for mitomycin C. Small sample sizes and inability to control pterygium size and grade limit these conclusions.
Methods: This 9-year retrospective study reviewed 119 primary pterygium eyes receiving pterygium excision at Wilmer eye institute (Baltimore, Maryland). The procedure data included the procedure type, adjuvants, attachment and surgeon experience (attending or trainee). Bare sclera, conjunctival autograft, amniotic membrane graft, and primary conjunctival closure techniques were analyzed for complications and recurrence.
Results: Average follow-up was 16.7 ± 22.0 months. The overall recurrence rates were: amniotic membrane 19% (n = 37), bare sclera 20% (n = 5), conjunctival autograft 16% (n = 68), and primary conjunctival closure 22% (n = 9). Statistically significant differences in recurrence rates between experience groups was only reached in the conjunctival autograft (p = 0.038, Fisher exact), but not after controlling for mitomycin C. Trainees used mitomycin C in conjunctival autograft cases more often (81% of cases versus 38% of attending cases, p = 0.001, Fisher Exact). No significant differences existed in recurrence time between groups (p = 0.164, log-rank). The average time to recurrence was 10.7 ± 12.0 months and 29% of recurrences occurred later than 12 months post-operatively. There were no statistically significant differences in complication rates.
Conclusion: A variety of techniques were utilized at an academic center and included amniotic membrane grafts, conjunctival autografts, bare sclera excision, and primary conjunctival closure. These procedures often used adjuvants like mitomycin C, or in a minority of cases used fibrin glue in place of, or in conjunction with sutures. In regards to the role of experience, trainees did not have statistically significant differences in complication rates and no significant difference in recurrence rates for any procedure after controlling for mitomycin C. Small sample sizes and inability to control pterygium size and grade limit these conclusions.
Keywords
Conjunctival disease; Ophthalmologic surgical procedures; Physician experience; Pterygium; Recurrence
BACKGROUND
A pterygium is a common fibrovascular growth extending from the conjunctiva onto the cornea. Pterygia are found worldwide and prevalence rates range from 1.2% in urban Melbourne to 23.4% for black individuals in Barbados [1-12]. Incidence studies have shown a 10-year incidence of 4.9 ± 0.4% in Beijing and 9-year incidence of 11.6% in Barbados [13,14]. The prevalence data remains controversial, but many studies suggest increased prevalence with increased age, male gender, rural residence, and ultraviolet light exposure [1-13,15]. Although widespread, much of pterygium pathogenesis remains a mystery.
Surgical treatment is often the choice for pterygium management. While lubricating drops and steroid drops may help symptoms, they do not prevent progression. The reasons for treatment can vary, but pterygium can cause significant discomfort, astigmatism, and vision change. Pterygia can cause the patient discomfort by creating foreign body sensations and also induces dry eye symptoms [16-20]. The astigmatism and vision change increases with more severe pterygium grade, but surgical excision of pterygia often reverses the astigmatism [2,21-23]. In addition to the discomfort and astigmatism, some patients may elect for surgery due to cosmetic concerns.
A persistent problem in the management of pterygia is they tend to recur after excision. Typically, recurrence occurs quickly, with a 50% chance within 4 months, and a 97% chance within 12 months [24]. With each recurrence, there is a gradual acceleration from 123 ± 113 days for the first recurrence to 67 ± 47 days for the third recurrence [24]. Risk factors for pterygia recurrence include having previous recurrences, fleshy non-translucent pterygia, and younger age [25-30].
Another variable in recurrence is the surgical procedure used. Bare sclera technique was one of the first surgical procedures for the treatment of pterygium and benefits from simplicity and speed, but suffers from the highest recurrence with rates ranging up to 88% [31]. Newer techniques utilize grafts, either amniotic membrane or autologous conjunctiva, and these have much lower recurrence rates [31]. The use of adjuvant therapy like mitomycin C, β-irradiation, or 5-fluorouracil can lower the rates of recurrence for these procedures, but may also increase severe adverse effects [31-35].
Despite the definitive treatment for pterygium being surgery, no consensus exists on which procedure is always appropriate. Outcomes like recurrence have been extensively analyzed based on the type of procedure and patient factors. However, very few studies have looked at how outcomes vary with surgeon experience. Many of the reports of recurrence for a given procedure vary, sometimes significantly, between studies. Some of this may be due to differences in the demographics or postoperative regimen, but there also is the possibility of the surgeon’s experience playing a role in outcomes. The newer techniques of grafting require more time and technical ability than the bare sclera technique. It may be surgeons in training have different outcomes than the experienced physicians. This factor of experience has not been well studied with pterygia. In one study, Farrah and Lee reported a statistical increase in both recurrence and complication rates in the trainee group for conjunctival autografts [36]. This study evaluated 45 consulting ophthalmologist primary pterygium cases and 129 trainee primary pterygium cases. Another study evaluated physicians performing conjunctival autografts and reported a range of 5-82% recurrence rates, with the physicians performing the most having the lowest recurrence rates [37]. The results of physician experience may have important consequences for the safety and outcomes of the patients. The objective of this study is to first analyze the variation in surgical technique selection at an academic teaching hospital. Second, the objective of the study was to examine how experience may play a role in surgical outcomes. Understanding this could help improve pterygium surgical outcomes, and thus improve vision.
Surgical treatment is often the choice for pterygium management. While lubricating drops and steroid drops may help symptoms, they do not prevent progression. The reasons for treatment can vary, but pterygium can cause significant discomfort, astigmatism, and vision change. Pterygia can cause the patient discomfort by creating foreign body sensations and also induces dry eye symptoms [16-20]. The astigmatism and vision change increases with more severe pterygium grade, but surgical excision of pterygia often reverses the astigmatism [2,21-23]. In addition to the discomfort and astigmatism, some patients may elect for surgery due to cosmetic concerns.
A persistent problem in the management of pterygia is they tend to recur after excision. Typically, recurrence occurs quickly, with a 50% chance within 4 months, and a 97% chance within 12 months [24]. With each recurrence, there is a gradual acceleration from 123 ± 113 days for the first recurrence to 67 ± 47 days for the third recurrence [24]. Risk factors for pterygia recurrence include having previous recurrences, fleshy non-translucent pterygia, and younger age [25-30].
Another variable in recurrence is the surgical procedure used. Bare sclera technique was one of the first surgical procedures for the treatment of pterygium and benefits from simplicity and speed, but suffers from the highest recurrence with rates ranging up to 88% [31]. Newer techniques utilize grafts, either amniotic membrane or autologous conjunctiva, and these have much lower recurrence rates [31]. The use of adjuvant therapy like mitomycin C, β-irradiation, or 5-fluorouracil can lower the rates of recurrence for these procedures, but may also increase severe adverse effects [31-35].
Despite the definitive treatment for pterygium being surgery, no consensus exists on which procedure is always appropriate. Outcomes like recurrence have been extensively analyzed based on the type of procedure and patient factors. However, very few studies have looked at how outcomes vary with surgeon experience. Many of the reports of recurrence for a given procedure vary, sometimes significantly, between studies. Some of this may be due to differences in the demographics or postoperative regimen, but there also is the possibility of the surgeon’s experience playing a role in outcomes. The newer techniques of grafting require more time and technical ability than the bare sclera technique. It may be surgeons in training have different outcomes than the experienced physicians. This factor of experience has not been well studied with pterygia. In one study, Farrah and Lee reported a statistical increase in both recurrence and complication rates in the trainee group for conjunctival autografts [36]. This study evaluated 45 consulting ophthalmologist primary pterygium cases and 129 trainee primary pterygium cases. Another study evaluated physicians performing conjunctival autografts and reported a range of 5-82% recurrence rates, with the physicians performing the most having the lowest recurrence rates [37]. The results of physician experience may have important consequences for the safety and outcomes of the patients. The objective of this study is to first analyze the variation in surgical technique selection at an academic teaching hospital. Second, the objective of the study was to examine how experience may play a role in surgical outcomes. Understanding this could help improve pterygium surgical outcomes, and thus improve vision.
METHODS
Study population
This study involved a retrospective chart review of pterygia patients seen at the Wilmer eye institute (Baltimore, Maryland, USA) in a 9-year period (January 2004-March 2013). One hundred forty-six patients were identified as receiving surgery for primary pterygium removal in their first eye by bare sclera excision, conjunctival autografting, limbal-conjunctival autografting, amniotic membrane grafting, or by primary conjunctival closure in the last 9 years. Twenty-seven cases (18.5%) without follow-up data beyond 14 days post-operative were removed. This group of 119 eyes will be referred to as the total-population.
For comparisons between attending and trainee surgeons, only attending surgeons who were involved in more than 10 pterygium surgeries in this time period were included. 96 eyes were then analyzed for recurrence analysis and will be referred to as the sub-population. Complication information was recorded from the paper charts. Of these 96 cases, 83 had obtainable paper charts in addition to electronic notes and were used in the complication rate analysis.
The patient information included race, age at operation, gender, and eye operated on. The information recorded about the procedure included the procedure type, amniotic membrane type, suture thickness and material, fibrin glue use, mitomycin C use, and the operating surgeon’s experience level (resident, fellow, or attending). These experience levels were grouped into two experience groups: attending and trainee. The trainee experience group included fellows and residents. Follow-up data included length of follow-up, time to recurrence, and post-operative complications. True corneal recurrence could not be determined retrospectively from many of the patient records, and therefore recurrence was recorded when any recurrence was documented by the physician. The post-operative complications were grouped into major and minor complications. Minor complications included graft edema, hemorrhage, subjective pain above 5 on a 0-10 scale, cyst, and irregular astigmatism. Major complications included graft retraction, symblepharon, granuloma, dellen, graft loss, and necrosis. Within each category of complications, occurrence of each complication was summed to create a complication rate for analysis.
Outcomes were recorded for each follow-up visit, and then combined into totals for the entire follow-up period for each patient. With regards to follow-up time in patients who underwent a procedure, had recurrence in the same eye, and then underwent repeat surgery, the follow-up length stopped for the first eye at the time of the second eye procedure.
The Johns Hopkins Medicine Institutional Review Board approved the study and research conformed to the Declaration of Helsinki.
For comparisons between attending and trainee surgeons, only attending surgeons who were involved in more than 10 pterygium surgeries in this time period were included. 96 eyes were then analyzed for recurrence analysis and will be referred to as the sub-population. Complication information was recorded from the paper charts. Of these 96 cases, 83 had obtainable paper charts in addition to electronic notes and were used in the complication rate analysis.
The patient information included race, age at operation, gender, and eye operated on. The information recorded about the procedure included the procedure type, amniotic membrane type, suture thickness and material, fibrin glue use, mitomycin C use, and the operating surgeon’s experience level (resident, fellow, or attending). These experience levels were grouped into two experience groups: attending and trainee. The trainee experience group included fellows and residents. Follow-up data included length of follow-up, time to recurrence, and post-operative complications. True corneal recurrence could not be determined retrospectively from many of the patient records, and therefore recurrence was recorded when any recurrence was documented by the physician. The post-operative complications were grouped into major and minor complications. Minor complications included graft edema, hemorrhage, subjective pain above 5 on a 0-10 scale, cyst, and irregular astigmatism. Major complications included graft retraction, symblepharon, granuloma, dellen, graft loss, and necrosis. Within each category of complications, occurrence of each complication was summed to create a complication rate for analysis.
Outcomes were recorded for each follow-up visit, and then combined into totals for the entire follow-up period for each patient. With regards to follow-up time in patients who underwent a procedure, had recurrence in the same eye, and then underwent repeat surgery, the follow-up length stopped for the first eye at the time of the second eye procedure.
The Johns Hopkins Medicine Institutional Review Board approved the study and research conformed to the Declaration of Helsinki.
STATISTICS
Data was analyzed using STATA 12 (StataCorp LP, College Station, Texas). Statistical significance was calculated using the chi-squared, Fisher exact test, or Mann-Whitney test, depending on the variable type and cell count. Time to recurrence was analyzed using a Kaplan-Meier curve and the log-rank test for statistical significance.
RESULTS
Total-population results
In regards to the results of the total-population of the 119 eyes, this study included 57 left eye cases and 62 right eye cases. The demographics of patients included 9 Asian, 12 Black, 12 Hispanic, 7 other, 3 unknown and 76 White. For the gender distribution, 54 female eyes and 65 male eyes underwent pterygium excision. The average age at the time of operation was 54.6 ± 12.5 years. The youngest patient treated for pterygium was 28.8 years old, and the oldest patient treated was 90.1 years old.
In the total-population data, there were 37 amniotic membrane grafts, 5 bare sclera, 68 conjunctival autograft, and 9 primary conjunctival closure operations performed. These procedures had an average follow up of 16.7 ± 22.0 months (range 0.5-95.8 months). In the attachment method, 96 cases used only sutures, 12 used fibrin glue only, and 6 used both. The use of fibrin glue was not associated with lower recurrence (p = 0.126, Fisher exact). Mitomycin C was used in 46 cases (39%). The recurrence rate with mitomycin C was 2.2% (1/46), while without mitomycin C was 27.4% (20/73). Mitomycin C was statistically significant in having lower recurrence (p=<0.001, Fisher exact). Recurrence rates were amniotic membrane 19%, bare sclera 20%, conjunctival autograft 16%, and primary conjunctival closure 22%. The procedure type was not statistically associated with recurrence rate (p=0.891, Fisher exact).
The time until recurrence averaged 10.7 ± 12.0 months with the fastest recurrence occurring in less than 2 weeks in a primary pterygium case excised by a bare sclera technique. The longest time to recurrence was 40.5 months in a primary pterygium patient excised with an amniotic membrane graft placed. Of the recurrences, 29% occurred later than 12 months post-operatively.
In the total-population data, there were 37 amniotic membrane grafts, 5 bare sclera, 68 conjunctival autograft, and 9 primary conjunctival closure operations performed. These procedures had an average follow up of 16.7 ± 22.0 months (range 0.5-95.8 months). In the attachment method, 96 cases used only sutures, 12 used fibrin glue only, and 6 used both. The use of fibrin glue was not associated with lower recurrence (p = 0.126, Fisher exact). Mitomycin C was used in 46 cases (39%). The recurrence rate with mitomycin C was 2.2% (1/46), while without mitomycin C was 27.4% (20/73). Mitomycin C was statistically significant in having lower recurrence (p=<0.001, Fisher exact). Recurrence rates were amniotic membrane 19%, bare sclera 20%, conjunctival autograft 16%, and primary conjunctival closure 22%. The procedure type was not statistically associated with recurrence rate (p=0.891, Fisher exact).
The time until recurrence averaged 10.7 ± 12.0 months with the fastest recurrence occurring in less than 2 weeks in a primary pterygium case excised by a bare sclera technique. The longest time to recurrence was 40.5 months in a primary pterygium patient excised with an amniotic membrane graft placed. Of the recurrences, 29% occurred later than 12 months post-operatively.
Sub-population results
When comparing the attending and trainee groups using the sub-population there were no statistically significant differences in the patient gender (p=0.466, chi-squared) race (p=0.199, Fisher exact), age (p=0.608, Mann-Whitney), or eye treated (0.393, Fisher exact). The demographic data are summarized in table 1.
| Variable | Attending cases | Trainee cases | Statistic |
| Patient gender | 53% Female (18/34) | 45% Female (28/62) | P=0.466(a) |
| Eye treated | 56% Left (19/34) | 47% Left (29/62) | P=0.393(a) |
| Average patient age | 52.8 ± 12.7 years (28.8-80.6) | 54.7 ± 12.7 years (29.9-90.1) | P=0.608(b) |
| Patient race | 71% Caucasian 0% Black 20% Hispanic 6% Asian 3% Other |
>68% Caucasian 10% Black 8% Hispanic 6% Asian 6% Other |
P=0.199(c) |
Attendings performed 34 of these procedures alone, while 62 had trainees involved in the procedure. Follow up time for attendings was 528 ± 697 days (range: 29-2917 days) and for trainees it was 439 ± 627 days (range: 17-2382 days). These follow up times were not different enough to reach statistical significance (p=0.128, Mann-Whitney)
Overall complication analysis included the 83 eyes with attainable paper charts. The other patients with absent paper charts were not included to avoid missing complications despite documented follow up in the electronic records. No complications were reported in 62 (75%) cases, 17 (20%) had one complication reported, 3 (4%) had two complications reported, and 1 (1%) had 3 complications reported. Only 5 had any major complications reported (6%). The breakdown of complications is seen in table 2. Complications reported included graft edema, granuloma, cyst, dellen, hemorrhage, graft retraction, significant pain and diplopia. There were no statistically significant differences in complication rates between experience groups (Mann-Whitney p-values all >0.208).
| Complication | All procedures | Amniotic membrane graft | Conjunctival autograft | |||
| Group | A | T | A | T | A | T |
| Number of patients(a) | 29 | 54 | 4 | 22 | 23 | 28 |
| Patients with any complications | 17% n = 4 |
31% n = 17 |
50% n = 2 |
50% n = 11 |
9% n = 2 |
21% n = 6 |
| Patients with a minor complication | 10% n = 2 |
28% n = 15 |
50% n = 2 |
41% n = 9 |
-- | 21% n = 6 |
| Patients with a major complication | 7% n = 2 |
6% n = 3 |
-- | 9% n = 2 |
9% n = 2 |
4% n = 1 |
| Graft edema | 3% n = 1 |
-- | 25% n = 1 |
-- | -- | -- |
| Granuloma | 3% n = 1 |
2% n = 1 |
-- | 5% n = 1 |
4% n = 1 |
-- |
| Cyst | -- | 2% n = 1 |
-- | -- | -- | 4% n = 1 |
| Dellen | 3% n = 1 |
-- | -- | -- | 4% n = 1 |
-- |
| Hemorrhage | -- | 9% n = 5 |
-- | 14% n = 3 |
-- | 7% n = 2 |
| Graft retraction | -- | 4% n = 2 |
-- | 5% n = 1 |
-- | 4% n = 1 |
| Significant pain | 3% n = 1 |
17% n = 9 |
25% n = 1 |
27% n = 6 |
-- | 11% n = 3 |
| Diplopia | -- | 2% n = 1 |
-- | 5% n = 1 |
-- | -- |
(a) All values are percentages except number of patients, which is a numerical count
There were statistically significant differences in techniques used between the two groups (p=0.036, Fisher exact). Table 3, summarizes the proportion of procedures chosen by the physician. Both groups used the conjunctival autograft procedure for the majority of their cases.
| Technique | Attending physician procedures performed |
Trainee procedures performed |
| Amniotic membrane graft | 18% (n = 6) | 42% (n = 26) |
| Bare sclera | 6% (n = 2) | 3% (n = 2) |
| Conjunctival autograft | 76% (n = 26) | 52% (n = 32) |
| Primary conjunctival closure | 0% (n = 0) | 3% (n = 2) |
| Total procedures | n = 34 | n = 62 |
When comparing the groups based on the type of procedure, there are no statistical differences in recurrence when using amniotic membrane techniques (p= >0.999, Fisher exact) or bare sclera (p= >0.999, Fisher exact). Conjunctival autografts had a statistical difference in recurrence rates (p=0.038, Fisher exact). There is trend for lower recurrence in conjunctival autografting by the trainees, but when controlling for mitomycin C use, the p-value rises for without mitomycin C (p=0.616, Fisher Exact). When using mitomycin C, there were no recurrences in the 10 attending conjunctival autografts and no recurrences in the 26 trainee conjunctival autografts. Trainees were more likely to use mitomycin C in conjunctival autograft cases, as they used it in 81% of cases versus 38% in attending cases and this was statistically different (p=0.001, Fisher Exact). The recurrence rates are summarized in table 4. Differences in the time to recurrence were not statistically significant (p=0.164, log-rank). The Kaplan-Meier curve is shown in figure 1.
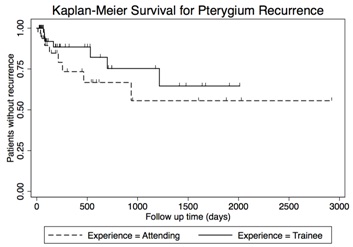
Figure 1: Ahmed Glaucoma Valve implant 6 months after surgery.
| Technique | Attending physician recurrences | Trainee procedures recurrences | P-value |
| Amniotic membrane graft | 1/6 (17%) | 5/26 (19%) | P = >0.999 |
| Amniotic membrane graft with MMC | 0/2 (0%) | 1/6 (17%) | P = >0.999 |
| Amniotic membrane graft without MMC | 1/4 (25%) | 4/20 (20%) | P = >0.999 |
| Bare sclera | 1/2 (50%) | P = >0.999 | |
| Conjunctival autograft | 6/26 (23%) | 1/32 (3%) | P = 0.038 |
| Conjunctival autograft with MMC | 0/10 (0%) | 0/26 (0%) | |
| Conjunctival autograft without MMC | 6/16 (38%) | 1/6 (17%) | P = 0.616 |
| Primary conjunctival closure | 0/0 (0%) | 0/2 (0%) | |
| Total | 8/34 (24%) | 6/62 (10%) | P = 0.064 |
DISCUSSION
This study aimed to record the variation in surgical technique selection at a teaching hospital, and examine how experience may play a role in surgical outcomes. With the excision of these pterygia at this teaching hospital, there were a variety of procedure types performed. Conjunctival autograft was performed in the majority of cases and for good reason. Conjunctival autograft is often regarded as the superior procedure because it provides good cosmesis and the lowest rates of recurrence [31,38]. However, the conjunctival autograft is not always the ideal procedure, and the variety of other techniques used in this study reflects this. Amniotic membrane grafts are very useful in patients with heavily scarred conjunctiva or when a patient may require glaucoma surgery [39,40]. Conjunctival autografts are often favored over the amniotic membrane grafts, as meta-analysis and clinical trials have found higher recurrence rates with the amniotic membrane technique [31,38,39,41,42]. In our results, amniotic membrane grafts had a 19% recurrence rate, while conjunctival autografts had a 16% recurrence rate. The primary conjunctival closure and bare sclera techniques are regarded as inferior because of their poor recurrence rates. Only 5 (4.2%) bare sclera and 9 (7.5%) primary conjunctival closure procedures were performed, which does support those procedures have fallen out of favor. However, these techniques despite their drawbacks are still valid techniques, and with a low risk of recurrence based on patient age and pterygium grade, they may provide satisfactory results. There certainly could be differences in recurrence rates that our study was unable to detect because of these small sample sizes.
As previously mentioned, preventing recurrence is a major goal of pterygium surgery. This prevents any future compromise to vision and avoids the additional cost and risk of recurrent pterygium surgery. Mitomycin C is one adjuvant often utilized to lower recurrence rates. The use of mitomycin C has been shown to lower recurrence rates in amniotic membrane grafts, bare sclera excisions, and conjunctival autografts [31-33,35,43]. In this study, the results were as expected, with the cases using mitomycin C having statistically significant lowering of recurrence rates. Mitomycin C certainly reduced the risk of recurrence, but mitomycin C also carries its own risk of vision threatening complications. Mitomycin C can produce scleral melting, secondary glaucoma, cataract, as well as other problems [32,34,35,44-48]. The known and unknown complications of mitomycin C requires cautious use despite the established lowering of recurrence rates [31-33,35,43]. None of the complications we report could specifically be attributed to mitomycin C.
Fibrin glue is a newer technology being implemented in pterygium surgery more recently, also with the goal of reducing recurrence rates. Many studies have found lower recurrence rates and faster operation times associated with fibrin glue use [29,49-54]. Our study population had only 18 cases using fibrin glue, and the recurrence rate was not statistically different from sutures (p=0.126, Fisher exact). Operation times were not recorded from this study and we could not assess the speed of fibrin glue or its learning curve in the trainees.
The other goal of this study was to determine any differences exist in recurrence or complication rates between trainees and attendings. It is important to note the statistical differences in the procedure types selected by each group, and that literature reported recurrence rates vary by the type of procedure [31]. Since the procedures have different recurrence rates, analyzing each procedure individually was important to isolate the variable of experience. When analyzing each procedure individually, no statistical differences were found in amniotic membrane grafts or bare sclera techniques. It was unlikely we would detect a difference with only four bare sclera cases. Primary conjunctival closure technique did not have any attending cases in which to compare the two trainee cases to. In addition, the number of patients in each group became small when dividing by procedure and controlling for adjuvants, so statistical power is low in all procedure types. Differences may exist, but our study was unable to detect them.
The conjunctival autograft technique did have a statistically significant difference in recurrence rates, and interestingly was lower in the trainee group. This is not likely due to experience, but likely due to the use of mitomycin C. The use of mitomycin C will lower recurrence rates and the trainees were statistically more likely to use mitomycin C than the attendings. Interestingly, while not statistically significant, there still was a trend for lower recurrence in the trainee group even after controlling for mitomycin C. Possibly, with two surgeons working, there may be improved outcomes from having an assistant, but this study did not specifically address this issue.
Farrah and Lee examined only conjunctival autograft procedures and reported higher complication rates and recurrence rates in the trainee group [36]. In Farrah and Lee’s study, they stated that in the trainee group, most operations involved the trainee alone [36]. With our trainee group, it could not be determined where the trainee remained the primary surgeon for the entire case or if instead, the attending performed critical components of the surgery. In all cases, no more than two surgeons were present (one attending and one trainee).
There were no statistically significant differences in complications for any of the procedures. However, there were trends for higher rates reported of hemorrhage, graft retraction, and significant pain in the trainee cases. This could be due to the inexperience of the trainee physician, a difference in compliance of postoperative management in resident patients, or it could be a bias in what the surgeon sees as significant and reports in the medical record. In, Farrah and Lee’s study they found higher complication rates in the trainee cases. The study of outcomes is important because complications are an integral component of patient safety. The outcomes of higher minor complications have to be weighed against the benefit of education.
A confounding factor we were unable to control is the pterygium size and grade. From previous literature, it is understood that pterygium size and grade increases the risk of recurrence [25-27]. In our cases, it cannot be determined if attendings selected the more complex and higher grade cases. If this holds true, the attendings’ recurrence rates may trend higher due to more difficult procedures and higher risk of recurrence. Younger age also increases the risk for recurrence, but age was not significantly different between the experience groups [25,28-30].
As with any retrospective review, follow-up bias exists. In 27 of 146 (18.5%) eligible primary pterygium cases in patients who had never received any pterygium surgeries followed up for less than two weeks and were excluded from analysis. These patients could have no problems, or conversely became discouraged with poor outcomes.
Another issue with our retrospective review is the non-standardized terminology of recurrence. Making the determination in many cases if the recurrence reported in the medical record represented a true corneal recurrence or an earlier stage was difficult. Here, prospective studies could record valuable data. A prospective trial could ensure consistent definitions of pterygium recurrence, avoids the retrospective study bias, and could record the degree of trainee involvement in the procedure.
As previously mentioned, preventing recurrence is a major goal of pterygium surgery. This prevents any future compromise to vision and avoids the additional cost and risk of recurrent pterygium surgery. Mitomycin C is one adjuvant often utilized to lower recurrence rates. The use of mitomycin C has been shown to lower recurrence rates in amniotic membrane grafts, bare sclera excisions, and conjunctival autografts [31-33,35,43]. In this study, the results were as expected, with the cases using mitomycin C having statistically significant lowering of recurrence rates. Mitomycin C certainly reduced the risk of recurrence, but mitomycin C also carries its own risk of vision threatening complications. Mitomycin C can produce scleral melting, secondary glaucoma, cataract, as well as other problems [32,34,35,44-48]. The known and unknown complications of mitomycin C requires cautious use despite the established lowering of recurrence rates [31-33,35,43]. None of the complications we report could specifically be attributed to mitomycin C.
Fibrin glue is a newer technology being implemented in pterygium surgery more recently, also with the goal of reducing recurrence rates. Many studies have found lower recurrence rates and faster operation times associated with fibrin glue use [29,49-54]. Our study population had only 18 cases using fibrin glue, and the recurrence rate was not statistically different from sutures (p=0.126, Fisher exact). Operation times were not recorded from this study and we could not assess the speed of fibrin glue or its learning curve in the trainees.
The other goal of this study was to determine any differences exist in recurrence or complication rates between trainees and attendings. It is important to note the statistical differences in the procedure types selected by each group, and that literature reported recurrence rates vary by the type of procedure [31]. Since the procedures have different recurrence rates, analyzing each procedure individually was important to isolate the variable of experience. When analyzing each procedure individually, no statistical differences were found in amniotic membrane grafts or bare sclera techniques. It was unlikely we would detect a difference with only four bare sclera cases. Primary conjunctival closure technique did not have any attending cases in which to compare the two trainee cases to. In addition, the number of patients in each group became small when dividing by procedure and controlling for adjuvants, so statistical power is low in all procedure types. Differences may exist, but our study was unable to detect them.
The conjunctival autograft technique did have a statistically significant difference in recurrence rates, and interestingly was lower in the trainee group. This is not likely due to experience, but likely due to the use of mitomycin C. The use of mitomycin C will lower recurrence rates and the trainees were statistically more likely to use mitomycin C than the attendings. Interestingly, while not statistically significant, there still was a trend for lower recurrence in the trainee group even after controlling for mitomycin C. Possibly, with two surgeons working, there may be improved outcomes from having an assistant, but this study did not specifically address this issue.
Farrah and Lee examined only conjunctival autograft procedures and reported higher complication rates and recurrence rates in the trainee group [36]. In Farrah and Lee’s study, they stated that in the trainee group, most operations involved the trainee alone [36]. With our trainee group, it could not be determined where the trainee remained the primary surgeon for the entire case or if instead, the attending performed critical components of the surgery. In all cases, no more than two surgeons were present (one attending and one trainee).
There were no statistically significant differences in complications for any of the procedures. However, there were trends for higher rates reported of hemorrhage, graft retraction, and significant pain in the trainee cases. This could be due to the inexperience of the trainee physician, a difference in compliance of postoperative management in resident patients, or it could be a bias in what the surgeon sees as significant and reports in the medical record. In, Farrah and Lee’s study they found higher complication rates in the trainee cases. The study of outcomes is important because complications are an integral component of patient safety. The outcomes of higher minor complications have to be weighed against the benefit of education.
A confounding factor we were unable to control is the pterygium size and grade. From previous literature, it is understood that pterygium size and grade increases the risk of recurrence [25-27]. In our cases, it cannot be determined if attendings selected the more complex and higher grade cases. If this holds true, the attendings’ recurrence rates may trend higher due to more difficult procedures and higher risk of recurrence. Younger age also increases the risk for recurrence, but age was not significantly different between the experience groups [25,28-30].
As with any retrospective review, follow-up bias exists. In 27 of 146 (18.5%) eligible primary pterygium cases in patients who had never received any pterygium surgeries followed up for less than two weeks and were excluded from analysis. These patients could have no problems, or conversely became discouraged with poor outcomes.
Another issue with our retrospective review is the non-standardized terminology of recurrence. Making the determination in many cases if the recurrence reported in the medical record represented a true corneal recurrence or an earlier stage was difficult. Here, prospective studies could record valuable data. A prospective trial could ensure consistent definitions of pterygium recurrence, avoids the retrospective study bias, and could record the degree of trainee involvement in the procedure.
CONCLUSION
In conclusion, we report a variety of pterygium excision techniques utilized in the last 9 years at a teaching hospital. Conjunctival autograft is often believed to be the superior procedure, but situations exist in which alternative techniques prove useful. In our data, the other procedures did not have significantly different recurrence rate outcomes. Additionally with these procedures, the cost and risk of utilizing adjuvants like mitomycin C or the use of fibrin glue should also be carefully weighted decisions. In regards to the role of physician experience, we were unable to determine if experience does play a role in outcomes of complication or recurrence rates. There was a difference in recurrence rate for the conjunctival autograft, but this likely represents differences in mitomycin C use and not surgical experience. There should also be an effort for prospective research studies to compare outcomes in all pterygium techniques based on surgeon experience. It may hold important implications in which techniques the less experienced physicians should perform to maximize patient safety and positive vision outcome.
REFERENCES
- Wong TY, Foster PJ, Johnson GJ, Seah SK, Tan DT (2001) The prevalence and risk factors for pterygium in an adult Chinese population in Singapore: the Tanjong Pagar survey. Am J Ophthalmol 131: 176-183.
- Gazzard G, Saw SM, Farook M, Koh D, Widjaja D, et al. (2002) Pterygium in Indonesia: prevalence, severity and risk factors. Br J Ophthalmol 86: 1341-1346.
- McCarty CA, Fu CL, Taylor HR (2000) Epidemiology of pterygium in Victoria, Australia. Br J Ophthalmol 84: 289-292.
- Landers J, Henderson T, Craig J (2011) Prevalence of pterygium in indigenous Australians within central Australia: the Central Australian Ocular Health Study. Clin Experiment Ophthalmol 39: 604-606.
- Tano T, Ono K, Hiratsuka Y, Otani K, Sekiguchi M, et al. (2013) Prevalence of pterygium in a population in northern Japan: the locomotive syndrome and health outcome in Aizu cohort study. Acta Ophthalmol 91: 232-236.
- Sun LP, Lv W, Liang YB, Friedman DS, Yang XH, et al. (2013) The prevalence of and risk factors associated with pterygium in a rural adult Chinese population: the Handan Eye Study. Ophthalmic Epidemiol 20: 148-154.
- Li Z, Cui H (2013) Prevalence and associated factors for pterygium in a rural adult population (the Southern Harbin eye study). Cornea 32: 806-809.
- Ang M, Li X, Wong W, Zheng Y, Chua D, et al. (2012) Prevalence of and racial differences in pterygium: a multiethnic population study in Asians. Ophthalmology 119: 1509-1515.
- Luthra R, Nemesure BB, Wu SY, Xie SH, Leske MC, et al. (2001) Frequency and risk factors for pterygium in the Barbados eye study. Arch Ophthalmol 119: 1827-1832.
- Fotouhi A, Hashemi H, Khabazkhoob M, Mohammad K (2009) Prevalence and risk factors of pterygium and pinguecula: the Tehran eye study. Eye (Lond) 23: 1125-1129.
- Ma K, Xu L, Jie Y, Jonas JB (2007) Prevalence of and factors associated with pterygium in adult Chinese: the Beijing eye study. Cornea 26: 1184-1186.
- Cajucom-Uy H, Tong L, Wong TY, Tay WT, Saw SM (2010) The prevalence of and risk factors for pterygium in an urban Malay population: the Singapore Malay Eye Study (SiMES). Br J Ophthalmol 94: 977-981.
- Zhao L, You QS, Xu L, Ma K, Wang YX, et al. (2013) 10-year incidence and associations of pterygium in adult Chinese: the Beijing eye study. Invest Ophthalmol Vis Sci 54: 1509-1514.
- Nemesure B, Wu SY, Hennis A, Leske MC, Barbados Eye Studies Group (2008) Nine-year incidence and risk factors for pterygium in the barbados eye studies. Ophthalmology 115: 2153-2158.
- Threlfall TJ, English DR (1999) Sun exposure and pterygium of the eye: a dose-response curve. Am J Ophthalmol 128: 280-287.
- Türky?lmaz K, Oner V, SevimMÅž, Kurt A, Sekeryapan B, et al. (2013) Effect of pterygium surgery on tear osmolarity. J Ophthalmol 2013: 863498.
- Julio G, Lluch S, Pujol P, Merindano D (2013) Ocular discomfort in pterygium patients. Optom Vis Sci 90: 269-274.
- Todani A, Melki SA (2009) Pterygium: current concepts in pathogenesis and treatment. Int Ophthalmol Clin 49: 21-30.
- Marcovich AL, Bahar I, Srinivasan S, Slomovic AR (2010) Surgical management of pterygium. Int Ophthalmol Clin 50: 47-61.
- Alpay A, U?urba? SH, Erdo?an B (2009) Comparing techniques for pterygium surgery. Clin Ophthalmol 3: 69-74.
- Lin A, Stern G (1998) Correlation between pterygium size and induced corneal astigmatism. Cornea 17: 28-30.
- Fong KS, Balakrishnan V, Chee SP, Tan DT (1998) Refractive change following pterygium surgery. CLAO J 24: 115-117.
- Kurna SA, Altun A, Aksu B, Kurna R, Sengor T (2013) Comparing treatment options of pterygium: limbal sliding flap transplantation, primary closing, and amniotic membrane grafting. Eur J Ophthalmol 23: 480-487.
- Hirst LW, Sebban A, Chant D (1994) Pterygium recurrence time. Ophthalmology 101: 755-758.
- Mahar PS, Manzar N (2014) The study of etiological and demographic characteristics of pterygium recurrence: a consecutive case series study from Pakistan. Int Ophthalmol 34: 69-74.
- Tan DT, Chee SP, Dear KB, Lim AS (1997) Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol 115: 1235-1240.
- Sandra S, Zeljka J, Zeljka VA, Kristian S, Ivana A (2014) The influence of pterygium morphology on fibrin glue conjunctival autografting pterygium surgery. Int Ophthalmol 34: 75-79.
- Varssano D, Shalev H, Lazar M, Fischer N (2013) Pterygium excision with conjunctival autograft: true survival rate statistics. Cornea 32: 1243-1250.
- Farid M, Pirnazar JR (2009) Pterygium recurrence after excision with conjunctival autograft: a comparison of fibrin tissue adhesive to absorbable sutures. Cornea 28: 43-45.
- Huerva V, March A, Martinez-Alonso M, Muniesa MJ, Sanchez C (2012) Pterygium surgery by means of conjunctival autograft: long term follow-up. Arq Bras Oftalmol 75: 251-255.
- Kaufman SC, Jacobs DS, Lee WB, Deng SX, Rosenblatt MI, et al. (2013) Options and adjuvants in surgery for pterygium: a report by the American Academy of Ophthalmology. Ophthalmology 120: 201-208.
- Lam DS, Wong AK, Fan DS, Chew S, Kwok PS, et al. (1998) Intraoperative mitomycin C to prevent recurrence of pterygium after excision: a 30-month follow-up study. Ophthalmology 105: 901-904.
- Fakhry MA (2011) The use of mitomycin C with autologous limbal-conjunctival autograft transplantation for management of recurrent pterygium. Clin Ophthalmol 5: 123-127.
- Mandour SS, Farahat HG, Mohamed HM (2011) Preoperative subpterygial mitomycin C injection versus limbal conjunctival autograft transplantation for prevention of pterygium recurrence. J Ocul Pharmacol Ther 27: 481-485.
- Song YW, Yu AH, Cai XJ (2010) Effectiveness of amniotic membrane transplantation combined with mitomycin C in the treatment of pterygium: a meta-analysis. Int J Ophthalmol 3: 352-355.
- Farrah JJ, Lee GA, Greenrod E, Vieira J (2006) Outcomes of autoconjunctival grafting for primary pterygia when performed by consultant compared with trainee ophthalmologists. Clin Experiment Ophthalmol 34: 857-860.
- Ti SE, Chee SP, Dear KB, Tan DT (2000) Analysis of variation in success rates in conjunctival autografting for primary and recurrent pterygium. Br J Ophthalmol 84: 385-389.
- Li M, Zhu M, Yu Y, Gong L, Zhao N, et al. (2012) Comparison of conjunctival autograft transplantation and amniotic membrane transplantation for pterygium: a meta-analysis. Graefes Arch Clin Exp Ophthalmol 250: 375-381.
- Ozer A, Yildirim N, Erol N, Yurdakul S (2009) Long-term results of bare sclera, limbal-conjunctival autograft and amniotic membrane graft techniques in primary pterygium excisions. Ophthalmologica 223: 269-273.
- Solomon A, Pires RT, Tseng SC (2001) Amniotic membrane transplantation after extensive removal of primary and recurrent pterygia. Ophthalmology 108: 449-460.
- Liang W, Li R, Deng X (2012) Comparison of the efficacy of pterygium resection combined with conjunctival autograft versus pterygium resection combined with amniotic membrane transplantation. Eye Sci 27: 102-105.
- Yu C, Liang W, Huang Y, Guan W (2011) Comparison of clinical efficacy of three surgical methods in the treatment of pterygium. Eye Sci 26: 193-196.
- Kareem AA, Farhood QK, Alhammami HA (2012) The use of antimetabolites as adjunctive therapy in the surgical treatment of pterygium. Clin Ophthalmol 6: 1849-1854.
- Ma DH, See LC, Liau SB, Tsai RJ (2000) Amniotic membrane graft for primary pterygium: comparison with conjunctival autograft and topical mitomycin C treatment. Br J Ophthalmol 84: 973-978.
- Safianik B, Ben-Zion I, Garzozi HJ (2002) Serious corneoscleral complications after pterygium excision with mitomycin C. Br J Ophthalmol 86: 357-358.
- Dougherty PJ, Hardten DR, Lindstrom RL (1996) Corneoscleral melt after pterygium surgery using a single intraoperative application of mitomycin-C. Cornea 15: 537-540.
- Bekibele CO, Ashaye A, Olusanya B, Baiyeroju A, Fasina O, et al. (2012) 5-Fluorouracil versus mitomycin C as adjuncts to conjunctival autograft in preventing pterygium recurrence. Int Ophthalmol 32: 3-8.
- Rubinfeld RS, Pfister RR, Stein RM, Foster CS, Martin NF, et al. (1992) Serious complications of topical mitomycin-C after pterygium surgery. Ophthalmology 99: 1647-1654.
- Kucukerdonmez C, Karalezli A, Akova YA, Borazan M (2010) Amniotic membrane transplantation using fibrin glue in pterygium surgery: a comparative randomised clinical trial. Eye (Lond) 24: 558-566.
- Ratnalingam V, Eu AL, Ng GL, Taharin R, John E (2010) Fibrin adhesive is better than sutures in pterygium surgery. Cornea 29: 485-489.
- Koranyi G, Seregard S, Kopp ED (2005) The cut-and-paste method for primary pterygium surgery: long-term follow-up. Acta Ophthalmol Scand 83: 298-301.
- Koranyi G, Artzén D, Wijk T (2013) Learning curve in the cut and paste method for surgery of primary pterygium. Acta Ophthalmol 91: 463-468.
- Pan HW, Zhong JX, Jing CX (2011) Comparison of fibrin glue versus suture for conjunctival autografting in pterygium surgery: a meta-analysis. Ophthalmology 118: 1049-1054.
- Koranyi G, Seregard S, Kopp ED (2004) Cut and paste: a no suture, small incision approach to pterygium surgery. Br J Ophthalmol 88: 911-914.
Citation: Janson BJ, Sikder S (2015) The Variation in Surgical Technique and Role of Physician Experience in Pterygium Surgical Outcomes. J Ophthalmic Clin Res 2: 011.
Copyright: © 2015 Ben J Janson, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2026, Copyrights Herald Scholarly Open Access. All Rights Reserved!

